-
Applications
-
Life Science
- rapidFLIMHiRes (Fluorescence Lifetime Imaging) New
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Foerster Resonance Energy Transfer (FRET)
- Pulsed Interleaved Excitation (PIE)
- scanning Fluorescence Correlation Spectroscopy (sFCS) New
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Dual-focus Fluorescence Correlation Spectroscopy (2fFCS)
- Stimulated Emission Depletion Microscopy (STED)
- Single Molecule Detection
- Time-resolved Fluorescence
- Fluorescence Anisotropy (Polarization)
- Pattern Matching Analysis
- Two-Photon Excitation (TPE)
- Diffuse Optical Tomography and Imaging
- Singlet Oxygen
- Laser Cutting/Ablation
- Materials Science
- Metrology
- Quantum Optics
-
Life Science
Life Science
Pulsed Interleaved Excitation (PIE)
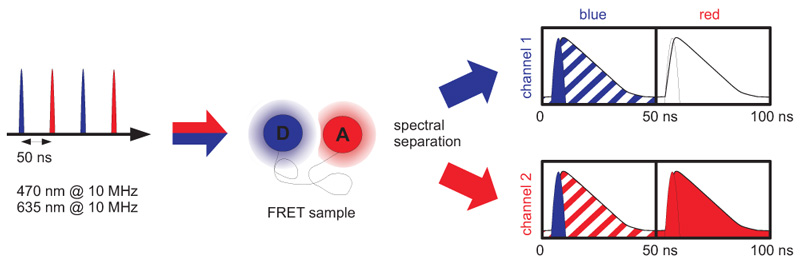
Technique enabling the synchronization of pulsed lasers on a nanosecond time scale for complex excitation patterns
Pulsed Interleaved Excitation (PIE) is a technique of synchronizing several pulsed lasers. The laser pulses are separated on a nanosecond time scale to allow simultaneous recording of the temporal behaviour of a sample molecule corresponding to each individual laser. For this method usually two lasers are chosen with suitable wavelengths for sequential excitation of both fluorophores. The laser pulses are delayed with respect to each other to yield a pulse sequence with interleaved pulses. To avoid crosstalk, the laser repetition rate is adjusted in that way that the fluorescence of each dye decays completely before excitation of the other fluorophore.
PIE is often used in combination with single-species Foerster Resonance Energy Transfer (spFRET) wherein donor and acceptor are excited alternately. In this way, the acceptor dye is excited independently of the FRET process to prove its existence and photoactivity. Molecules lacking an active donor or acceptor are separated from active FRET complexes. This allows to differentiate a FRET molecule, even with a very low FRET efficiency, from a molecule with an absent or non-fluorescing acceptor.
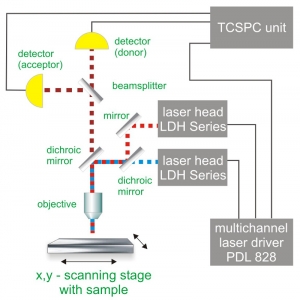 PIE experiments require at least two pulsed lasers and a dedicated multi-channel laser driver. In that way arbitrary burst patterns can be generated, which is extremely useful for e.g. multicolor excitation schemes.
PIE experiments require at least two pulsed lasers and a dedicated multi-channel laser driver. In that way arbitrary burst patterns can be generated, which is extremely useful for e.g. multicolor excitation schemes.
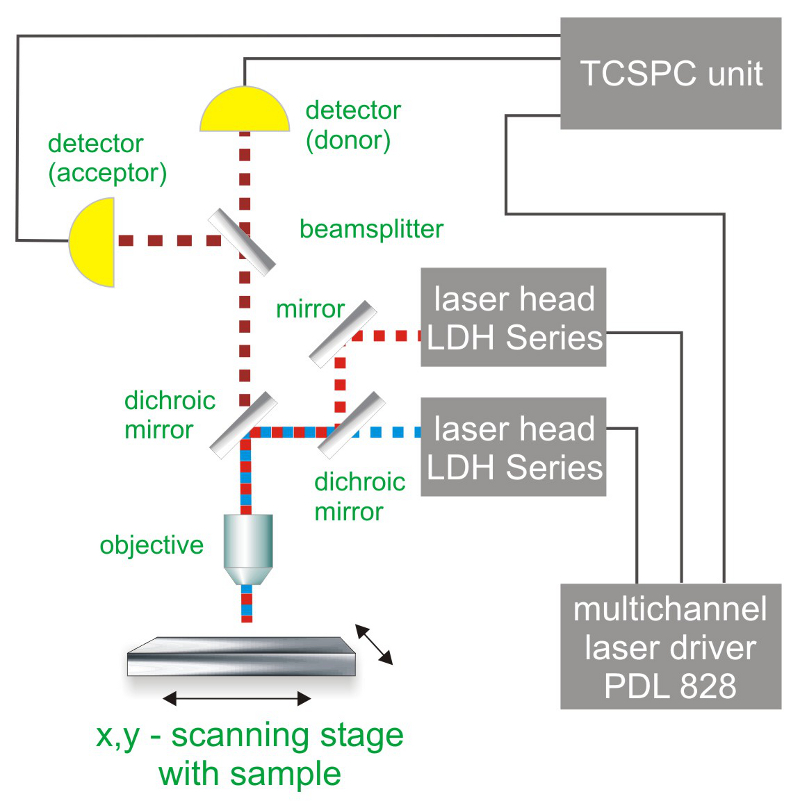
Consequently the essential components of a PIE set-up are:
- pulsed laser source (picosecond diode lasers)
- dedicated multi-channel laser driver
- single photon sensitive detector
- dichroic mirror (to separate fluorescence signal from excitation light)
- objective (to focus the excitation light into the sample and collect fluorescence signal)
- TCSPC unit to measure the time between excitation and fluorescence emission
- scanner (optional)
Related technical and application notes:
Pulsed Interleaved Excitation (PIE) can be added to the following PicoQuant microscopy products
Single Photon Counting Confocal Microscope
Luminosa combines state-of-the-art hardware with cutting edge software to deliver highest quality data while simplifying daily operation. The software includes context-based workflows for FLIM, FRET, and FCS, which allow the users to focus on their samples. Tight integration of hardware and software enable automated routines, e.g., for auto-alignment or laser power calibration, which increase ease of use as well as reproducibility of experiments. Still, an open mode of operation is available for full access to every optomechanical component via software.
Modular, time-resolved confocal fluorescence microscopy platform
The MicroTime 200 time-resolved fluorescence microscope system is a powerful instrument capable of Foerster Resonance Energy Transfer (FRET), Fluorecence Corelation Spectroscopy and its daughter techniques as well as Fluorescence Lifetime Imaging (FLIM) with single molecule detection sensitivity. It contains the complete optics and electronics for recording virtually all aspects of the fluorescence dynamics of microscopic samples or femtoliter volumes. The instrument gains its exceptional sensitivity and flexibility in combination with unprecedented ease-of-use from a unique fusion of miniaturized and highly sophisticated state-of-the-art technologies. Although, these technologies enable to run an instrument of comparable complexity and power without having to spend more time on instrument maintenance than on original scientific content, the MicroTime200 remains an open platform that allows the advanced scientist to easily built upon the open character of the instrument in order to realize highly customized applications
LSM Upgrade Kits from PicoQuant add time-resolved capabilities to laser scanning microscopes from many major manufactures
Confocal Laser Scanning Microscopes (LSMs) are widely used tools in cell and molecular biology, biochemistry and other related sciences. PicoQuant's LSM Upgrade Kits greatly enhance the capabilities of these microscopes by extension to time-resolved techniques, and thereby providing not only Foerster Resonance Energy Transfer (FRET) or Fluorescence Lifetime Imaging (FLIM) but also Fluorescence Correlation Spectroscopy (FCS) and a wealth of other time resolved techniques. The LSM Upgrade Kit combines PicoQuant products to a ready-to-use kit that fits your specific application on a state of the art Laser Scanning Microscope of your choice from Leica, Nikon, Olympus or Zeiss.
Upright time-resolved confocal microscope
The upright time-resolved microscope MicroTime 100 contains the complete optics and electronics for recording FRET, FLIM, FCS as well as fluorescence decays in small volumes by means of Time-Correlated Single Photon Counting (TCSPC). The system is based on a conventional upright microscope body that permist easy access to a wide range of sample shapes and sizes.
 The following core components are needed to build a system capable of PIE-FRET measurements, which are (partly) available from PicoQuant:
The following core components are needed to build a system capable of PIE-FRET measurements, which are (partly) available from PicoQuant:
- Laser drivers
- Laser or LED heads
- Photon Counting Detector
- TCSPC and MCS Electronics
- Analysis software
- Scanning device
- Galvo- or Piezo-Scanner
- Confocal optics
PIE is a very useful tool in FRET experiments. For studies resolving molecular distances in the nanometer scale, FRET can be enhanced using PIE to eliminate contributions from incomplete FRET pairs with missing or non fluorescing acceptors. The analysis of single-species FRET (spFRET) measurements is often complicated by the fact that not all molecules under study are necessarily marked with both donor and acceptor molecule, but often lack e.g. the acceptor molecule. Additional complications arise from multiple labeled species, giving not only one but multiple FRET processes. A differentiation between these single species is realized by applying PIE, but is effectively only possible on the single molecule level. The underlying approach is based on pulsed interleaved excitation of both the acceptor and the donor molecule. With PIE, the three subpopulations donor only, acceptor only and complete FRET molecules can be distinguished.
Furthermore, the concentrations of all three species can be derived using time-gated FCS after burst selection. It is therefore in principle possible to deduce all important parameters necessary for the FRET efficiency calculation (e.g. cross-talk of the donor emission into the acceptor channel and direct excitation of the acceptor) from a single measurement containing a mixture of all three subpopulations. This is of great importance under conditions where control measurements can not easily be performed, e.g. in living cells. The method can even be extended for excitation with more than two wavelengths for complete analysis of triple FRET or FRET in combination with the detection of further, fluorescently labeled molecules.
PIE is also a good complementation of Fluorescence Cross-Correlation (FCCS) experiments in the presence of FRET. In this way, the method can be applied to determine the concentrations of all three subpopulations donor only, acceptor only and complete FRET molecules (see above)
In addition, PIE allows for an excellent separation of different dyes due to the dye-specific excitation wavelengths which is also applicable for measurements performed in liquids (e.g. FCS). This results in enhanced sensitivity and elimination of crosstalk in FCS and FCCS experiments.
Furthermore, PIE is used for simultaneous multi-color excitation with a single detection channel.
As a variant of PIE, pulse trains can be generated in phosphorescence lifetime measurements to accelerate the acquisition time.
Pulsed Interleaved Excitation used for single molecule spectroscopy: Revealing the structural architecture of Prothrombin
 Prothrombin is a plasma protein that circulates at a concentration of 0.1 mg/ml. It is composed of 622 aa, organized in 4 domains and 3 linkers. Crystallization of prothrombin has succeeded only recently and required deletion of the Gla domain or significant portions of the flexible Lnk2. An unexpected molecular picture has emerged from these structures: prothrombin is organized as two rigid ends, the N-terminal Gla domain/kringle-1 pair and the C-terminal kringle-2/protease domain pair, capable of different relative arrangements mediated by Lnk2. Here we apply single molecule Förster resonance energy transfer (smFRET) to investigate the structural architecture and dynamics of prothrombin in solution.
Prothrombin is a plasma protein that circulates at a concentration of 0.1 mg/ml. It is composed of 622 aa, organized in 4 domains and 3 linkers. Crystallization of prothrombin has succeeded only recently and required deletion of the Gla domain or significant portions of the flexible Lnk2. An unexpected molecular picture has emerged from these structures: prothrombin is organized as two rigid ends, the N-terminal Gla domain/kringle-1 pair and the C-terminal kringle-2/protease domain pair, capable of different relative arrangements mediated by Lnk2. Here we apply single molecule Förster resonance energy transfer (smFRET) to investigate the structural architecture and dynamics of prothrombin in solution.
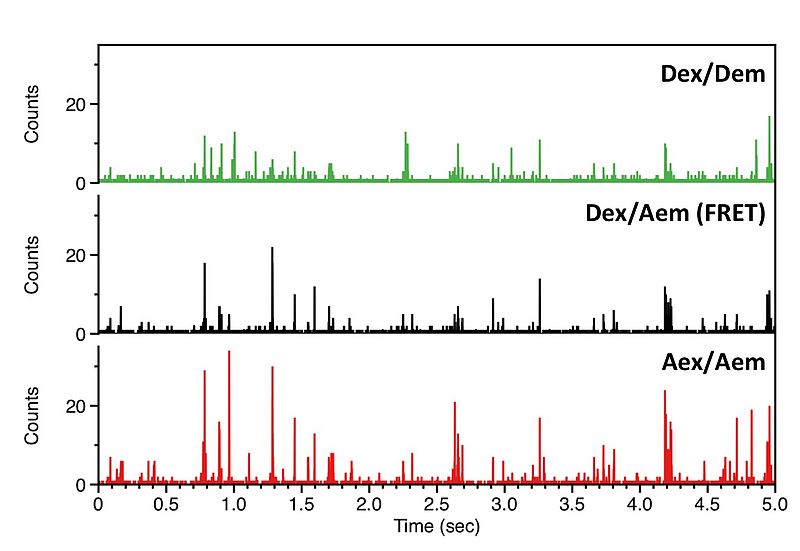
We record the fluorescence of molecules freely diffusing through a femtoliter observation volume defined by a focused laser beam and confocal optics. Our experiments are carried out with pulsed interleaved excitation (PIE), which reports the status of both donor and acceptor fluorophores by sorting molecules on the basis of relative donor:acceptor stoichiometry (S) and apparent FRET efficiency (E).
The conformation of full-length prothrombin in solution was investigated with FRET couples positioned in the N-terminal Gla domain/kringle-1 pair (34/101), the C-terminal kringle-2/protease domain pair (210/478), or across the flexible Lnk2 (101/478 and 120/478). Only the FRET couples positioned across Lnk2 report two distinct populations of interprobe distances. The flexibility of Lnk2 produces relative arrangements of the rigid ends of the zymogen in solution not captured by our recent X-ray structural studies. Using information from existing crystal structures of prothrombin and the interprobe distances measured by smFRET we built a model of the closed conformation of prothrombin in solution that shows the intramolecular collapse of kringle-1 onto the protease domain mediated by the Y93-W547.
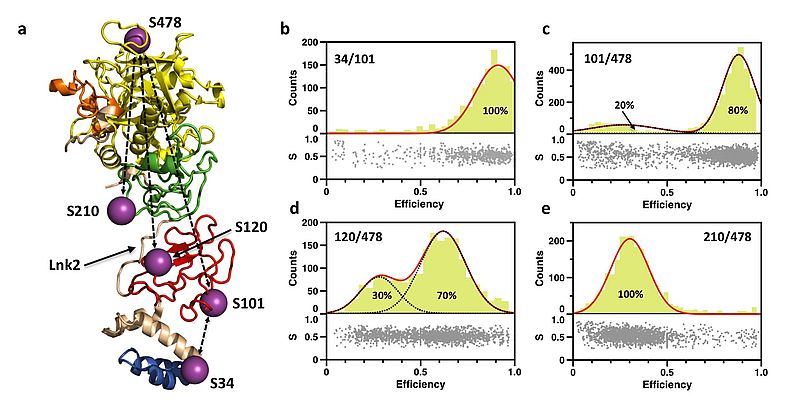 Prothrombin exists in equilibrium between two alternative conformations, open and closed, that differ 50 Å in length. The closed conformation predominates (70%) and features an unanticipated intramolecular collapse of Y93 in kringle-1 onto W547 in the protease domain that obliterates access to the active site.
Prothrombin exists in equilibrium between two alternative conformations, open and closed, that differ 50 Å in length. The closed conformation predominates (70%) and features an unanticipated intramolecular collapse of Y93 in kringle-1 onto W547 in the protease domain that obliterates access to the active site.
Set-up:
- MicroTime 200
- Excitation: 532 nm and 638 nm, 40 MHz repetition rate, PIE
- Emission: dual channel detection with 550 - 590 nm (donor) and 655 – 725 nm (acceptor)
- Analysis: SymPhoTime 64
References: Pozzi N et al., J Biol Chem. (2016), 291, 18107-18116; Pozzi N et al., J Biol Chem. (2016) 291, 6071-6082
PIE-FRET experiment enables monitoring of Mg2+ driven RNA folding of a tetraloop receptor
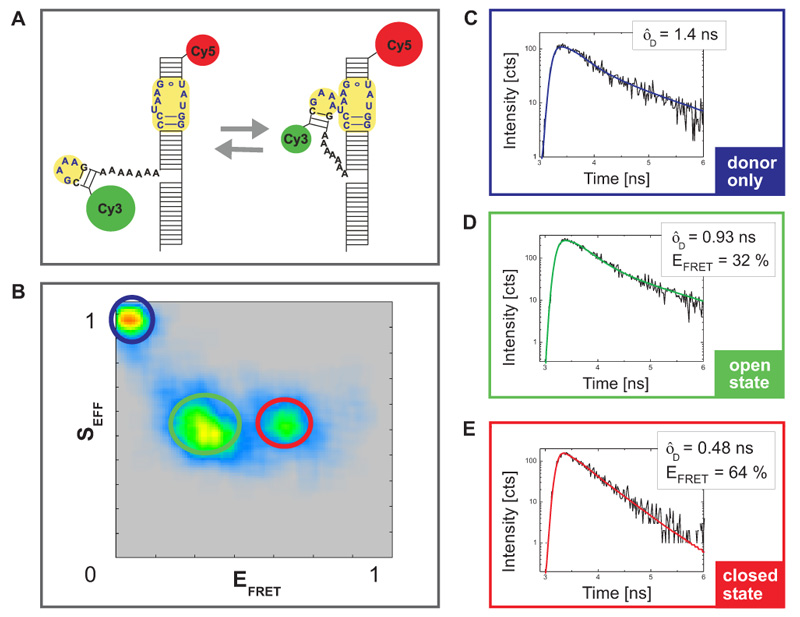
The analyzed construct contains an important RNA folding motif consisting of a tetraloop receptor labeld with CY5 as acceptor fluorophore and a flexible tetraloop fused to the donor CY3 (Fig. A). The RNA folding is Mg2+ driven and can be monitored by FRET as this construct could adopt two different conformations: an open state without any interaction between tetraloop and receptor and a closed state wherein due to the interaction between the two regions the donor and acceptor dye come into very close vicinity. This leads to FRET and a quenching of the donor fluorescence intensity and lifetime.
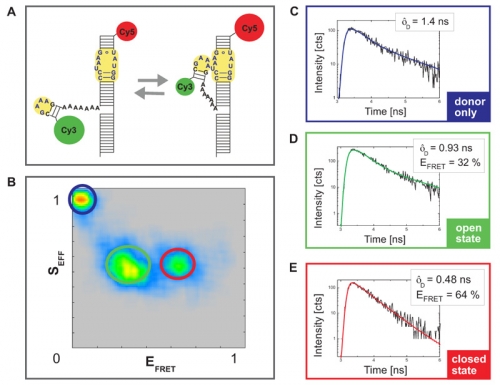 Fig. B shows a plot of the FRET efficiency E versus the Photon stoichiometry S that clearly displays three subpopulations within the sample (for details of the analysis see S. Weiss, Biophys. J. 88 (2005) 2939). S is the ratio between the number of FRET photons and donor photons (nominator) and the number of all photons (denominator) and is calculated as:
Fig. B shows a plot of the FRET efficiency E versus the Photon stoichiometry S that clearly displays three subpopulations within the sample (for details of the analysis see S. Weiss, Biophys. J. 88 (2005) 2939). S is the ratio between the number of FRET photons and donor photons (nominator) and the number of all photons (denominator) and is calculated as:
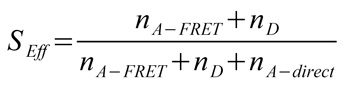
S is decreasing towards smaller numbers if the acceptor is present and can directly be excited via PIE. Thus, the population with a higher value of S and almost zero FRET efficiency corresponds to donor only labeled molecules. The other two populations have a similar photon stoichiometry but different FRET efficiencies.
For each sub-population the donor fluorescence lifetime was fitted by a 2-exponential decay model (Fig. C-E). The longer lifetime component was used to determine the FRET efficiency. The sub-population with the lower E corresponded to the open configuration whereas the molecules exhibiting a closed configuration and interaction between tetraloop and tetraloop receptor showed high FRET efficiency.
Set-up:
- MicroTime 200
- Excitation: 532 nm, 40 MHz repetition rate
- Emission: dual channel detection with 550 - 620 nm (donor) and 660 – 740 nm (acceptor)
- Analysis: SymPhoTime
In collaboration with J. Fiore and David Nesbitt, JILA, University of Colorado, Boulder, USA
Reference: J. Fiore et al., Biochem. (2009), 48, 02550-02558
Using two pulsed lasers for PIE mode for single molecule FRET investigations of Phosphoglycerate Kinase
Single-molecule FRET (smFRET) studies of phosphoglycerate kinase from yeast (yPGK) were performed at different concentrations of guanidine hydrochloride (GndHCl) to investigate protein folding.
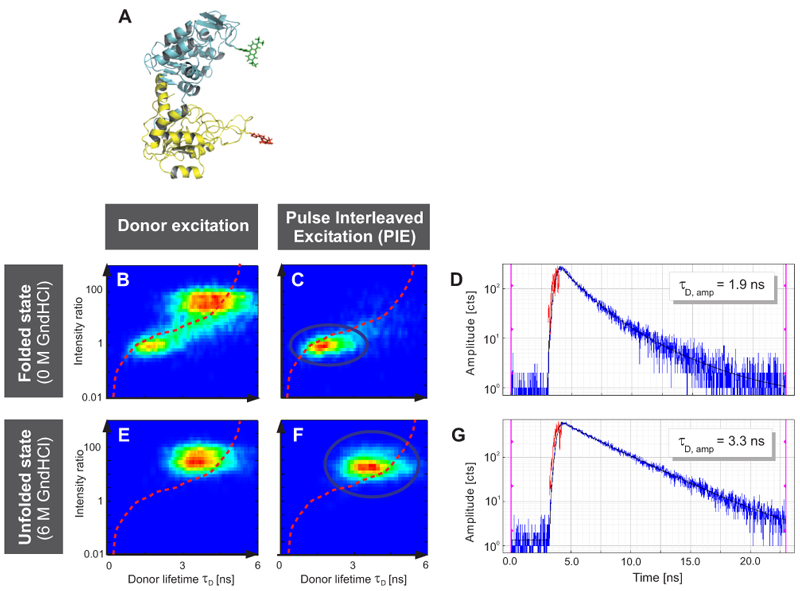
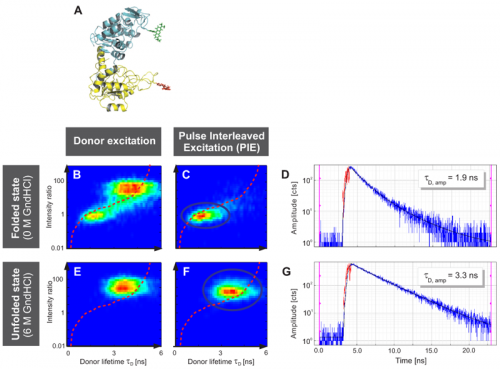 Fig. A displays the three-dimensional structure of yPGK and the fluorophores Alexa 488 and Alexa 647 attached to position 135 in the N-terminal (cyan) and position 209 in the C-terminal (yellow) domain, respectively. This FRET pair displays a Foerster radius R0 of 48.9 Å.
Fig. A displays the three-dimensional structure of yPGK and the fluorophores Alexa 488 and Alexa 647 attached to position 135 in the N-terminal (cyan) and position 209 in the C-terminal (yellow) domain, respectively. This FRET pair displays a Foerster radius R0 of 48.9 Å.
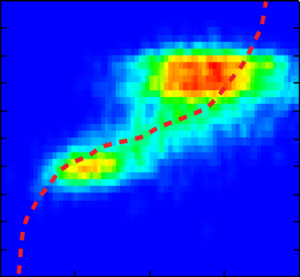
Interdomain distances of the native state and evolving unfolded states at increasing GndHCl concentrations were analyzed. The conformational characterization of native and unfolded states was performed by measuring FRET efficiencies for individual protein molecules at different GndHCl concentrations. The results of the Multiparameter Fluorescence Detection (MFD) with the depiction of the FRET indicator FD/FA versus the donor lifetime τD are shown (Fig. B, C, E and F). At 0 M GndHCl mainly the native population with high FRET was visible (Fig. B and C). The fitted donor fluorescence lifetime decreased to 1.9 ns (Fig. D). Without PIE, the donor only population was additionally observed (Fig. B).
An unfolded state population with no or low FRET and significantly expanded structure compared to the native state was detected at 6 MGndHCl concentration (Fig. E and F). Without PIE it is not possible to differentiate between this unfolded state and the donor only population (Fig. E). After exclusion of the donor only subpopulation via PIE (Fig. F), the unfolded protein configuration could be analyzed and a donor fluorescence lifetime of 3.3 ns was fitted (Fig. G).
The red lines indicate the theoretical prediction between the FRET indicator FD/FA and the donor lifetime τD in the presence of the acceptor2 taking into account distinct fluorescence quantum yields and detection efficiencies. Thus, the combination of PIE with MFD enabled for quantitative smFRET experiments.
Set-up:
- MicroTime 200
- Excitation: 470 nm and 640 nm, 20 MHz repetition rate, PIE
- Emission: dual channel detection with 500 - 540 nm (donor) and 655 – 725 nm (acceptor)
- Analysis: SymPhoTime
Data courtesy of by J. Fitter, Research Centre Jülich, Germany.
References:
[1] Rosenkranz T., Schlesinger R., Gabba M., Fitter J., ChemPhysChem 12, p.0704-0710 (2011)
[2] Sisamakis E., Valeri A., Kalinin S., Rothwelllow P. J., Seidel C. A. M., Methods in Enzymology, Vol.475, p.455-514 (2010)
Latest 10 publications related to Pulsed Interleaved Excitation (PIE)
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this application and our products in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.

 Contact us
Contact us Luminosa
Luminosa MicroTime 200
MicroTime 200 LSM Upgrade Kit
LSM Upgrade Kit MicroTime 100
MicroTime 100