-
Applications
-
Life Science
- rapidFLIMHiRes (Fluorescence Lifetime Imaging) New
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Foerster Resonance Energy Transfer (FRET)
- Pulsed Interleaved Excitation (PIE)
- scanning Fluorescence Correlation Spectroscopy (sFCS) New
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Dual-focus Fluorescence Correlation Spectroscopy (2fFCS)
- Stimulated Emission Depletion Microscopy (STED)
- Single Molecule Detection
- Time-resolved Fluorescence
- Fluorescence Anisotropy (Polarization)
- Pattern Matching Analysis
- Two-Photon Excitation (TPE)
- Diffuse Optical Tomography and Imaging
- Singlet Oxygen
- Laser Cutting/Ablation
- Materials Science
- Metrology
- Quantum Optics
-
Life Science
Life Science
Stimulated Emission Depletion Microscopy (STED)
Imaging beyond the optical diffraction limit
Stimulated emission depletion microscopy (STED) is a fluorescence microscopy technique that overcomes the diffraction limited resolution of confocal microscopes. The resolution enhancement is essentially based on switching off the fluorescence of dye molecules by stimulated emission using intense laser light in the outer regions of the diffraction limited excitation focus. This intense radiation causes almost all of the excited molecules to return to the ground state. Fluorescence from the remaining excited dye molecules in the center of the excitation focus is then detected and used to form the high resolution images. An even further resolution enhancement is possible by applying time gates to the collected data (gated STED or gSTED). As STED creates an effectively smaller observation volume, it can also be applied to other methods such as FCS. In that case, collecting data at different observation volume diameters can help to disentangle complex 2D diffusion scenarios in heterogeneous samples such as biological membranes.
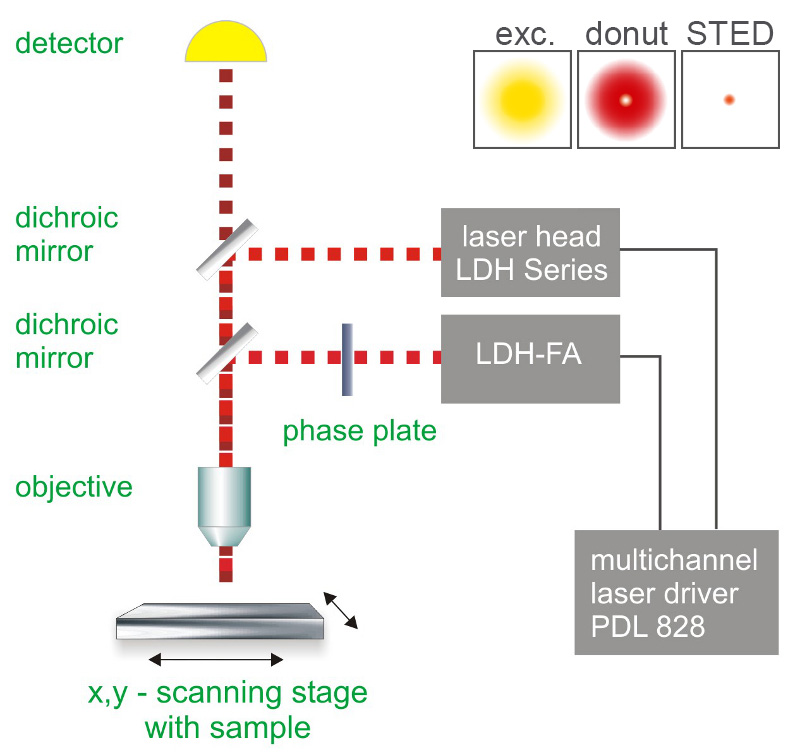
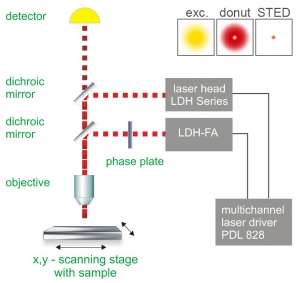 The STED method uses pairs of synchronized laser pulses. The first laser pulse generated by, e.g. a picosecond pulsed diode laser, is used to excite the fluorescence dye and produces an ordinary diffraction limited focus. The excitation pulse is immediately followed by a depletion pulse, which is red-shifted in frequency to the emission spectrum of the dye. By spatially arranging the STED pulse in a doughnut shape using specially designed phase plates, only the fluorescence from molecules at the periphery of the excitation focus is quenched via stimulated emission. In the center of the doughnut, where the STED laser intensity is zero, fluorescence remains unaffected and is detected by single-photon sensitive detectors (e.g., Single Photon Avalanche Diodes, SPADs).
The STED method uses pairs of synchronized laser pulses. The first laser pulse generated by, e.g. a picosecond pulsed diode laser, is used to excite the fluorescence dye and produces an ordinary diffraction limited focus. The excitation pulse is immediately followed by a depletion pulse, which is red-shifted in frequency to the emission spectrum of the dye. By spatially arranging the STED pulse in a doughnut shape using specially designed phase plates, only the fluorescence from molecules at the periphery of the excitation focus is quenched via stimulated emission. In the center of the doughnut, where the STED laser intensity is zero, fluorescence remains unaffected and is detected by single-photon sensitive detectors (e.g., Single Photon Avalanche Diodes, SPADs).
Consequently the essential components of a STED microscope set-up are:
- pulsed laser source for excitation and depletion
- single photon sensitive detector
- dichroic mirror (to overlay excitation and depletion lasers and to separate fluorescence signal from excitation light)
- phase plate for transforming the STED pulse into a doughnut shape
- objective
- data acquisition unit, e.g., a TCSPC module
- suited scanning system
Nobel Prize winner Stefan W. Hell talks about the origins of STED microscopy
PicoQuant offers a complete, turn-key solution for STED super-resolution microscopy
Time-resolved confocal fluorescence microscope with super-resolution capability
The MicroTime 200 STED is a powerful instrument capable of STED microscopy, Fluorecence Corelation Spectroscopy and its daughter techniques as well as Fluorescence Lifetime Imaging (FLIM) with single molecule detection sensitivity. It contains the complete optics and electronics for recording virtually all aspects of the fluorescence dynamics of microscopic samples or femtoliter volumes and with a spatial resolution below 50 nm. The instrument gains its exceptional sensitivity and flexibility in combination with unprecedented ease-of-use from a unique fusion of miniaturized and highly sophisticated state-of-the-art technologies. Although, these technologies enable to run an instrument of comparable complexity and power without having to spend more time on instrument maintenance than on original scientific content, the MicroTime 200 STED remains an open platform that allows the advanced scientist to easily built upon the open character of the instrument in order to realize highly customized applications.
 The following core components are needed to build a system capable of STED measurements, which are (partly) available from PicoQuant:
The following core components are needed to build a system capable of STED measurements, which are (partly) available from PicoQuant:
- Laser drivers
- Laser or LED heads
- Depletion laser head
- Phase plate
- Photon Counting Detector
- Data acqusition units such as TCSPC Electronics
- Scanning device
- Galvo- or Piezo-Scanner
- Analysis software
- Confocal optics
Correlative FLIM-FRET and STED imaging reveals protein interactions and structure in vivo
 The investigation of minute protein structures and interactions within nanoscopic cellular structures in living cells is challenging but essential for understanding biological processes.
The investigation of minute protein structures and interactions within nanoscopic cellular structures in living cells is challenging but essential for understanding biological processes.
FLIM-FRET allows detecting and quantifying protein interactions. These interactions occur within a few nm, but localizing protein pairs is diffraction-limited to approximately 200 nm. STED enables imaging with sub-diffraction resolution down to below 50 nm. Therefore, the correlation of FLIM-FRET and STED images facilitates studying protein interactions within sub-diffraction structures.
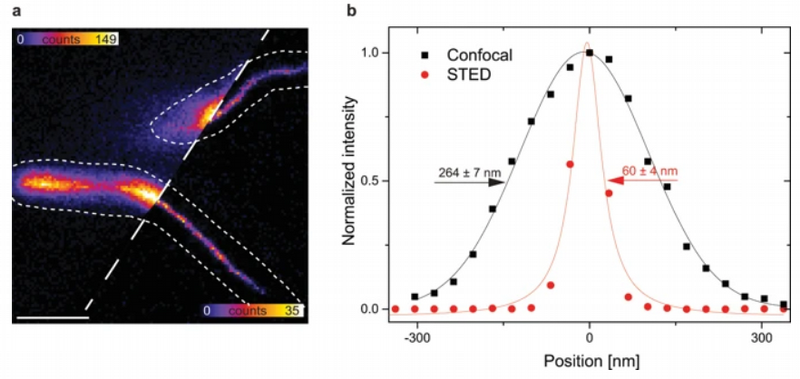
Magnetotactic bacteria intracellularly mineralize magnetic nanoparticles, called magnetosomes. These are collected into a chain along a cytoskeletal filament formed by the actin-like MamK protein. The linker protein MamJ attaches the magnetosomes to the filament.
In this example, the mechanical stability of the MamJ-MamK pair was tested in vivo. FLIM-FRET was used to monitor the connection between the filament and the magnetosome membrane via MamJ. STED images allowed checking for filament fragmentation after application of an external rotating magnetic field.
The far-red fluorescent protein TagRFP657 proved suitable for STED imaging, permitting a 4-fold reduction in apparent filament diameter from 240 nm to about 60 nm.
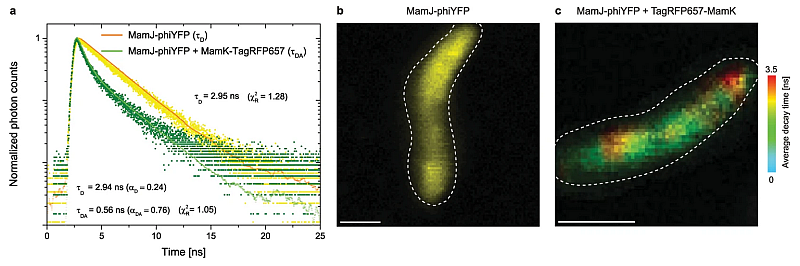
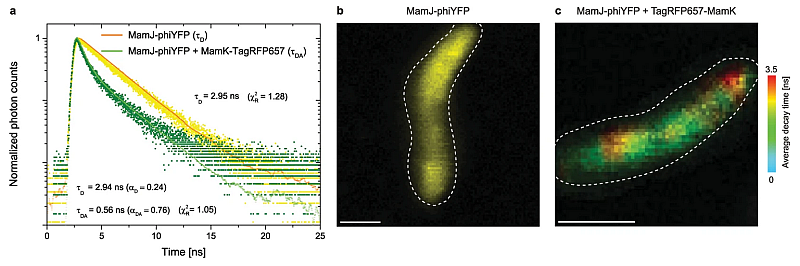 To enable acquisition of correlated FLIM-FRET and STED images in vivo, a new FRET pair was developed, which consists of the fluorescent proteins phiYFP and TagRFP657. Fluorescence decay curves show that the decay time of the donor MamJ-phiYFP decreases from 2.95 ns to 0.56 ns in the presence of the acceptor TagRFP657-MamK, which is an indication of MamJ-MamK interaction.
To enable acquisition of correlated FLIM-FRET and STED images in vivo, a new FRET pair was developed, which consists of the fluorescent proteins phiYFP and TagRFP657. Fluorescence decay curves show that the decay time of the donor MamJ-phiYFP decreases from 2.95 ns to 0.56 ns in the presence of the acceptor TagRFP657-MamK, which is an indication of MamJ-MamK interaction.
Set-up:
- MicroTime 200 with custom-built STED add-on including an LDH-P-FA-765 as depletion laser
- Excitation: 475 nm (donor), 635 nm (acceptor)
- Emission: 500 – 550 nm (donor), 655 – 725 nm (acceptor)
- Analysis: SymPhoTime 64
Images adapted and reproduced under CC BY license from Nature Publishing Group
Reference: Günther et al., Sci Rep 9, 19615 (2019)
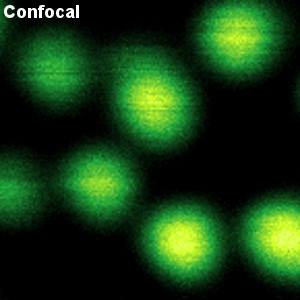
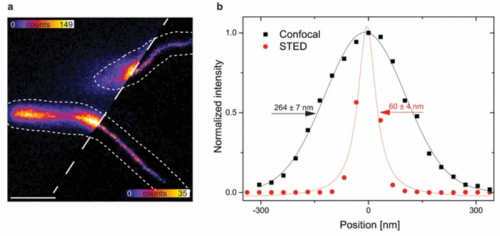
Demonstrating the enhancement of spatial resolution of STED with Crimson beads
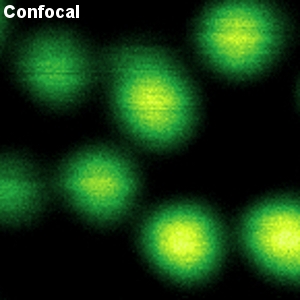
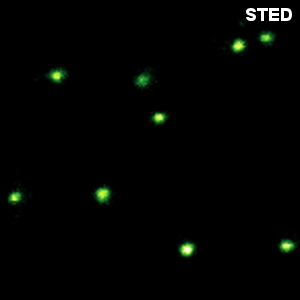
The example shows a comparison between confocal and STED images of Crimson beads (20 nm diameter). Fluorescent beads are often used to evaluate the resolution of a microscope. The FWHM in the confocal image is 260 nm, and in the STED image it is 38 nm. The resolution enhancement is clearly visible.
Set-up:
- MicroTime 200 STED
- Excitation: 640 nm
- Depletion: 765 nm
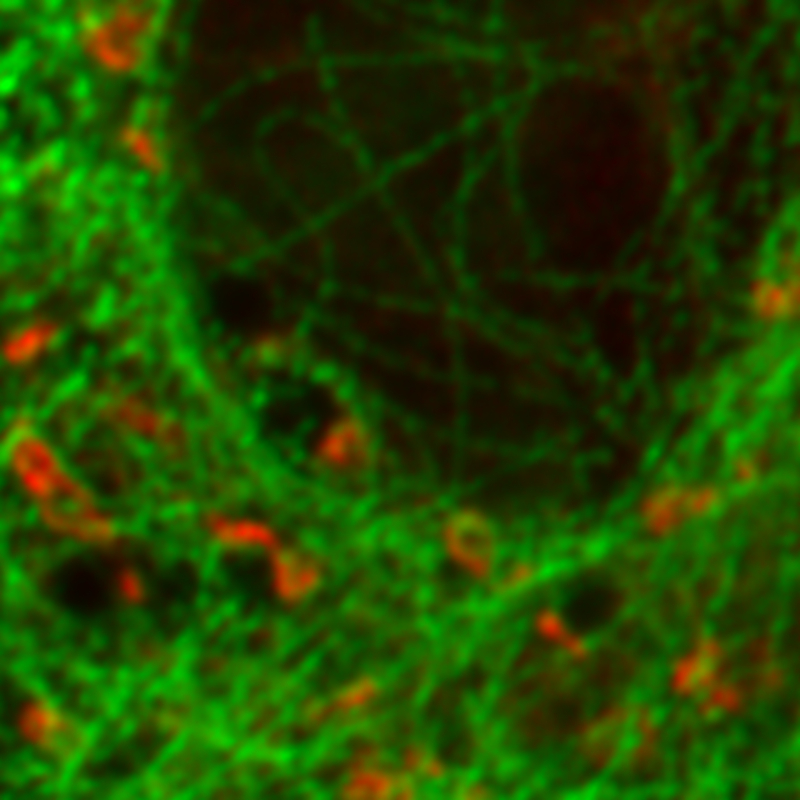
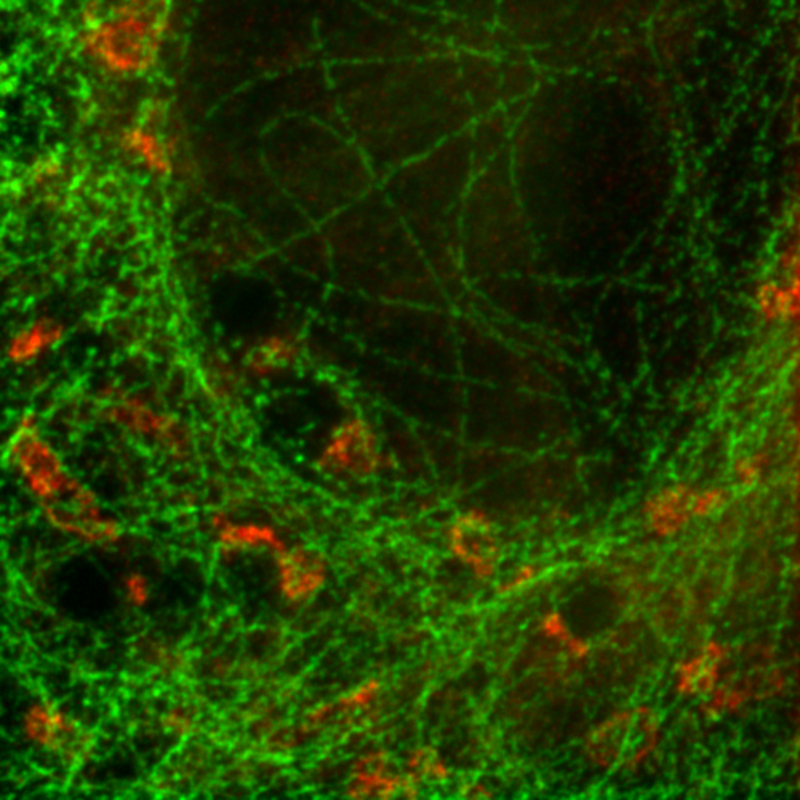
Combining STED with PIE and pattern matching for dual species separation
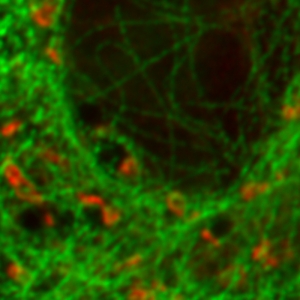
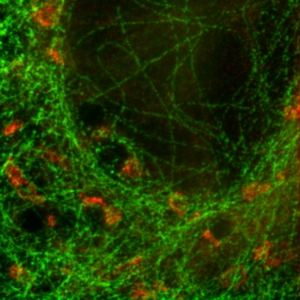
Two fluorescent species can be distinguished by pulsed interleaved excititation (PIE) with two slightly shifted wavelengths and detection of the fluorescence in two spectral bands. Fluorescence pattern matching was used to identify the contributions of each fluorophore. The example shows part of a U2OS cell labeled for tubulin with Abberior STAR 635p (green) and giantin with Atto647N (red) utilizing a single STED laser wavelength and excitation with two different diode lasers at 640 nm and 660 nm.
Set-up:
- MicroTime 200 STED
- Excitation 640 nm and 660 nm
- Depletion: 765 nm
Sample courtesy of Markus Sauer, University of Würzburg, Germany
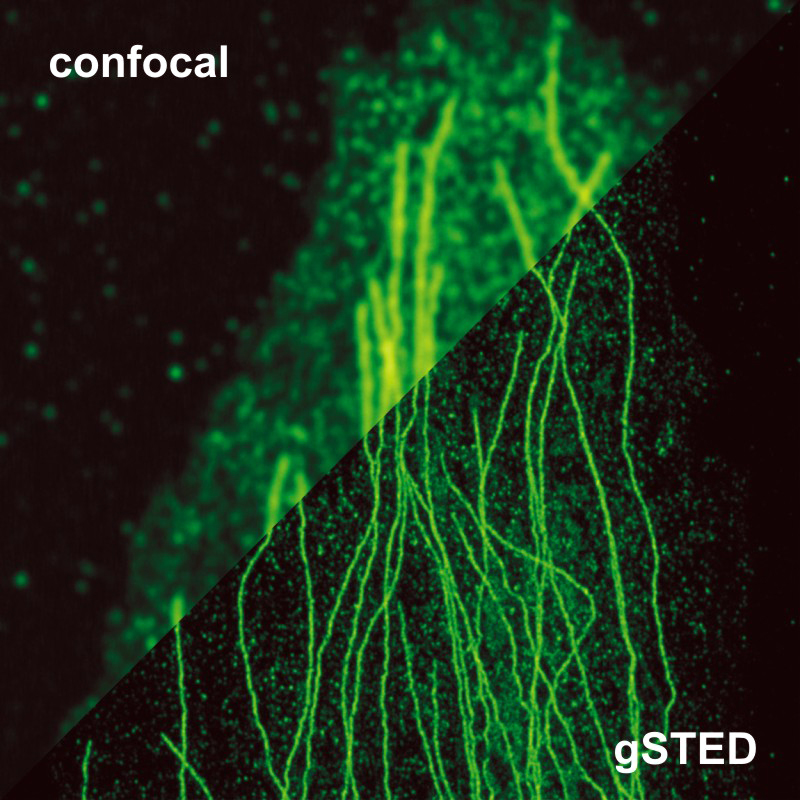
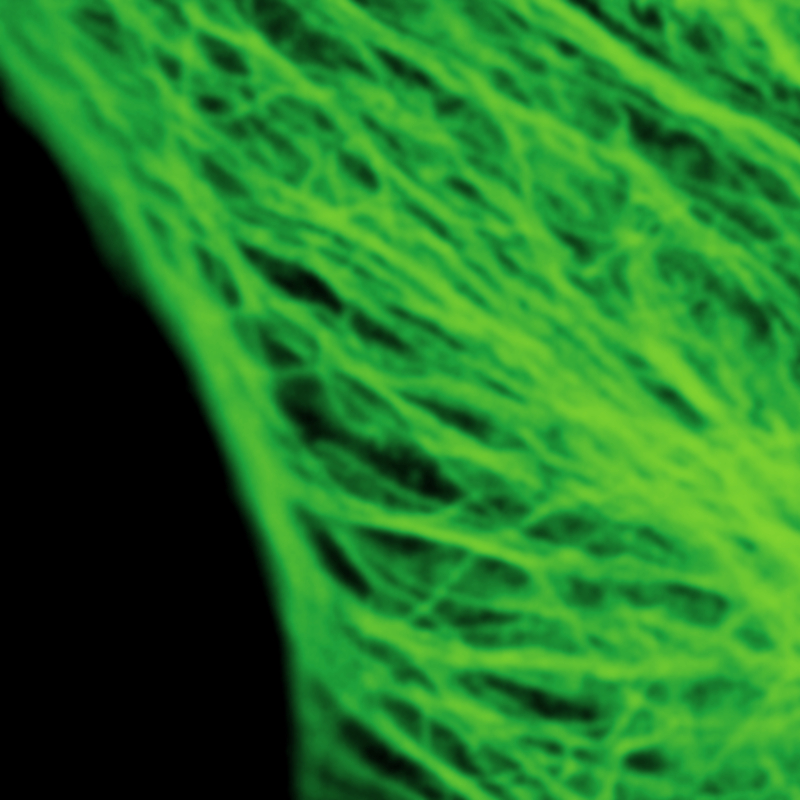
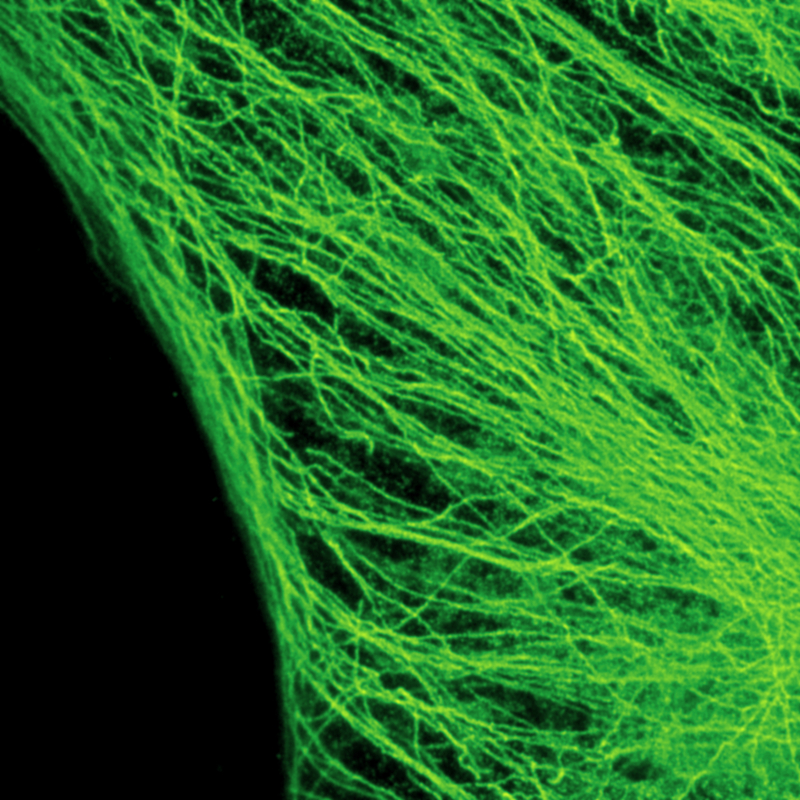
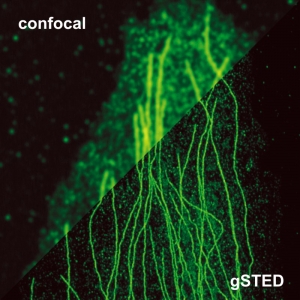
Stimulated emission depletion micrscopy provides a clear view of a cell’s cytoskeleton
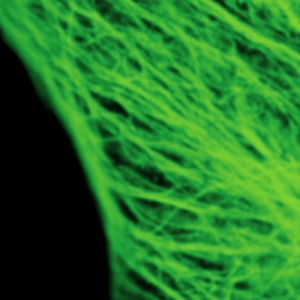
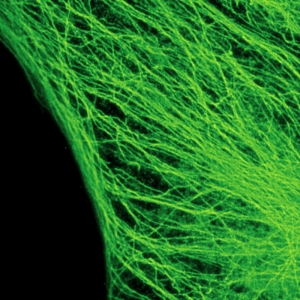
The example shows tubulin structures in a HeLa cell labeled with Abberior STAR635p. Super-resolution permits the clearer distinction of the filamentous structures in the STED image.
Set-up:
- MicroTime 200 STED
- Excitation: 640 nm
- Depletion: 765 nm


Specifically labeled DNA origami is now commonly used to evaluate the resolution of diffraction-limit-breaking microscopes. Here, origami with two rows of 11 dye molecules with a distance of 71 nm was used. Image size: 4 µm x 4µm
Set-up:
- MicroTime 200 STED
- Excitation: 640 nm
- Depletion: 765 nm
The samples were provided by GATTAquant GmbH and the Tinnefeld lab, TU Braunschweig, Germany
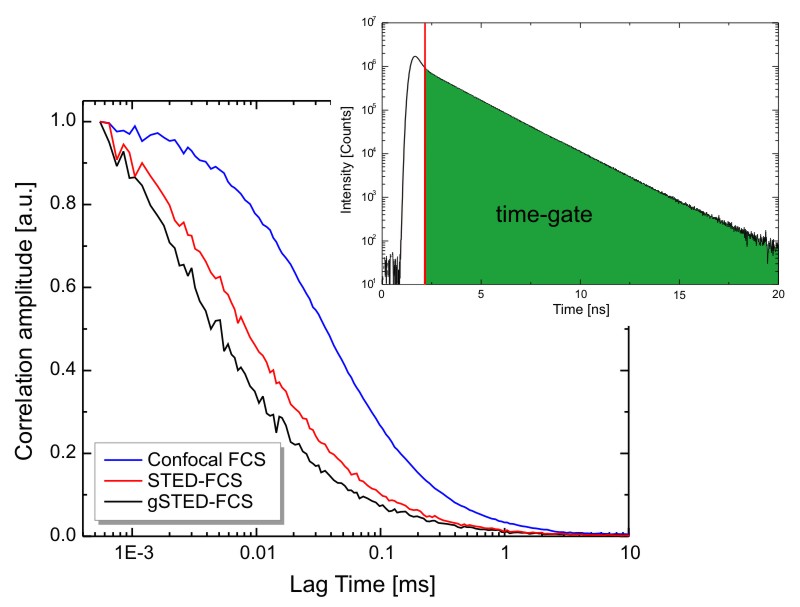
Time gated Stimulated Emission Depletion (gSTED) improves Florescence Correlation Spectrocsopy (FCS)
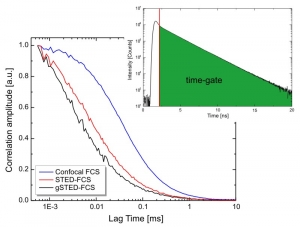 The example shows FCS of Atto655 in water using the standard confocal (blue) and STED (red) configuration. Due to the smaller detection volume in the STED experiment, the FCS curve is shifted to shorter lag times. By applying a time-gate that rejects early photons, the observation volume is decreased even more, leading to a further shift of the FCS curve towards shorter lag times (gSTED, black)
The example shows FCS of Atto655 in water using the standard confocal (blue) and STED (red) configuration. Due to the smaller detection volume in the STED experiment, the FCS curve is shifted to shorter lag times. By applying a time-gate that rejects early photons, the observation volume is decreased even more, leading to a further shift of the FCS curve towards shorter lag times (gSTED, black)
Set-up:
- MicroTime 200 STED
- Excitation: 640 nm
- Depletion: 765 nm
Using STED microscopy to image Crimson beads
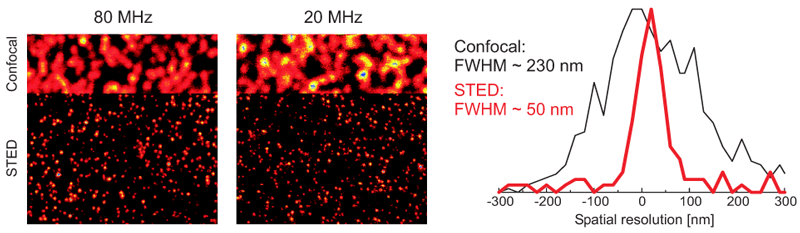
The example shows a comparison between confocal and STED images of Crimson beads (20 nm diameter) recorded at different repetition rates of the two lasers. The beads were excited using the LDH-P-C-640B and the LDH-P-FA-765 was used as STED laser. The upper parts of the images shows the beads imaged with a standard confocal setup, whereas the lower parts show the STED images of the same sample region. The resolution enhancement is clearly visible in the plotted cross-sections through one bead, with the STED images reaching a spatial resolution of approx. 50 nm.
Images courtesy of A. Honigmann, C. Eggeling and S.W. Hell, MPI Göttingen
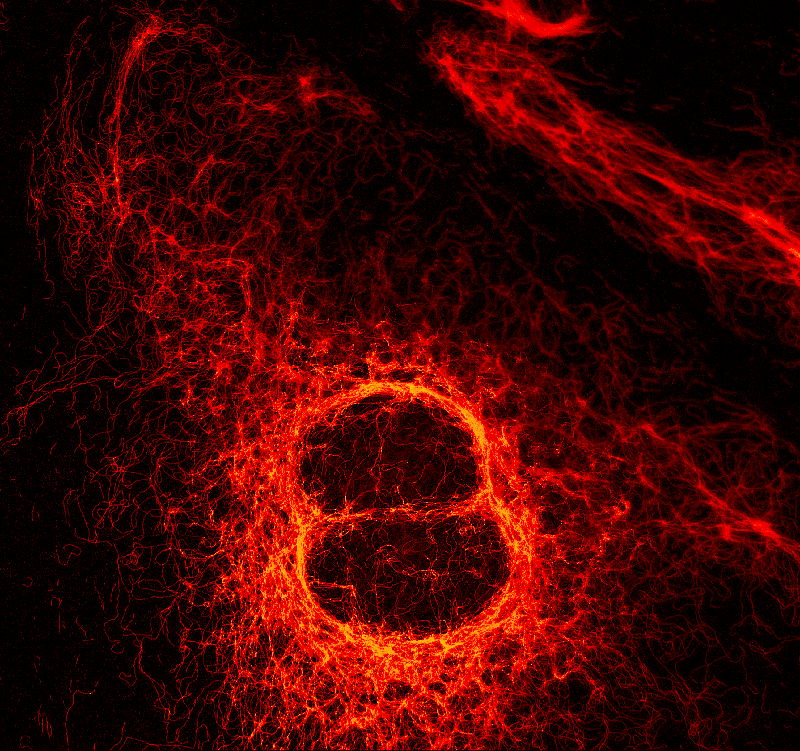
Imaging vimentin fibers with SETD super-resolution microscopy
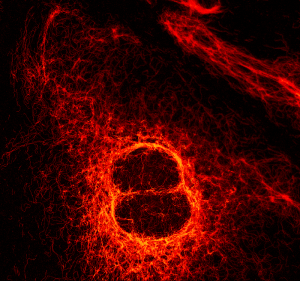 The example shows vimentin fibers stained with ATTO 565 via primary and secondary antibodies. The Solea laser has been used to excite the sample at 560 nm. A lateral resolution of approx. 50 nm is obtained, which can be expected for a fiber with a diameter of 10 nm, covered by antibodies with a diameter of approx. 8 nm each.
The example shows vimentin fibers stained with ATTO 565 via primary and secondary antibodies. The Solea laser has been used to excite the sample at 560 nm. A lateral resolution of approx. 50 nm is obtained, which can be expected for a fiber with a diameter of 10 nm, covered by antibodies with a diameter of approx. 8 nm each.
Data courtesy of J. Engelhardt and S.W. Hell, DKFZ Heidelberg
Latest 10 publications related to STED
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this application and our products in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.

 Contact us
Contact us MicroTime 200 STED
MicroTime 200 STED