Fluorescence System Upgrades
LSM Upgrade Kit
Compact FLIM and FCS Upgrade Kit for Laser Scanning Microscopes
- FLIM, FRET, and FCS in one turn-key system
- Compact, easy-to-use and maintenance-free kit, customized to all major LSMs in various configurations for unlimited flexibility
- Highest sensitivity with up to 4 detection channels
- Fluorescence lifetimes from < 100 ps up to µs
- Advanced and user-friendly data analysis software with multiple analysis tools
- Options for anisotropy measurements and deep-tissue FLIM imaging
- rapidFLIMHiRes - visualize dynamic processes with ultra fast FLIM imaging and outstanding 5 ps time resolution
Confocal Laser Scanning Microscopes (LSMs) are widely used tools in biochemistry, cell biology, and other related life sciences. The capabilities of these microscopes can be further enhanced by using time-resolved techniques, granting following advantages:
- Lifetime FRET for quantitative measurements of FRET efficiency
- Time-resolved imaging reveals environmental parameters such as ion concentrations or pH
- Lifetime measurements are independent from fluorophore concentration
- Separation of molecules with spectrally overlapping emission by lifetime fingerprinting
- Reduced number of needed detectors - one detector is sufficient for simultaneous detection of different fluorophores based on their specific lifetimes by pattern matching
- Discrimination of fluorescence light against elastic and Raman scattering and other background contributions by temporal resolution
- Decay time as a further parameter enhances the accuracy of analytical measurements
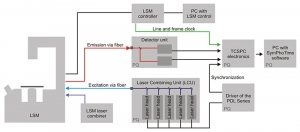 The Upgrade Kit is an external addition to the LSM and grants easy-to-use, versatile, and comfortable performance without time-consuming adjustments. The turn-key setup consists of three major parts: picosecond pulsed excitation, single molecule sensitive detection, and Time-Correlated Single Photon Counting (TCSPC) data acquisition.
The Upgrade Kit is an external addition to the LSM and grants easy-to-use, versatile, and comfortable performance without time-consuming adjustments. The turn-key setup consists of three major parts: picosecond pulsed excitation, single molecule sensitive detection, and Time-Correlated Single Photon Counting (TCSPC) data acquisition.
Flexible excitation system
 All samples containing commonly used fluorophores can be examined with the LSM Upgrade Kit. For this purpose, the excitation subsystem consists of a pulsed diode laser driver of the PDL Series and different laser heads with pulses in the picosecond time regime (additional CW mode is available as an option).
All samples containing commonly used fluorophores can be examined with the LSM Upgrade Kit. For this purpose, the excitation subsystem consists of a pulsed diode laser driver of the PDL Series and different laser heads with pulses in the picosecond time regime (additional CW mode is available as an option).
The available wavelengths range from 375 to 900 nm. The laser heads are integrated along with multiple optical components in one Laser Combining Unit (LCU) for comfortable handling and coupling into an optical fiber enabling single or multicolor excitation. The sample background is significantly reduced due  to the narrow excitation spectra of the lasers.
to the narrow excitation spectra of the lasers.
The repetition rate and laser power can be adapted via the laser driver to the fluorescence lifetime which minimizes bleaching of the sample. As an alternative to pulsed diode lasers, especially for multi-photon excitation schemes, short pulse laser systems such as Titanium:Sapphire lasers can be integrated as well.
Excellent timing with picosecond resolution
 Fluorescence lifetimes down to a few picoseconds or even up to milliseconds for phosphorescence and luminescence studies are easily resolvable with the LSM Upgrade Kit. This broad measurement range covers almost all samples that are analyzed in life as well as material sciences. Time-resolved microscopy requires the registration of not only the photons themselves, but also their position in time and, for imaging, in space. The ideal technique for that purpose is the Time-Tagged Time-Resolved (TTTR) data acquisition developed by PicoQuant, which is a variation of the classical method of Time-Correlated Single Photon Counting (TCSPC). Using TTTR data acquisition vastly different measurement procedures, like FLIM, lifetime based FRET, FCS, or even coincidence correlation ("antibunching") can be performed, based on just one fundamental data format. The TTTR format ist supported by all available TCSPC electronics from PicoQuant. Different modules with up to eight independent TCSPC channels for fast and parallel detection are available which provide highest data throughput, multi-stop possibility, and low dead time to realize short acquisition times.
Fluorescence lifetimes down to a few picoseconds or even up to milliseconds for phosphorescence and luminescence studies are easily resolvable with the LSM Upgrade Kit. This broad measurement range covers almost all samples that are analyzed in life as well as material sciences. Time-resolved microscopy requires the registration of not only the photons themselves, but also their position in time and, for imaging, in space. The ideal technique for that purpose is the Time-Tagged Time-Resolved (TTTR) data acquisition developed by PicoQuant, which is a variation of the classical method of Time-Correlated Single Photon Counting (TCSPC). Using TTTR data acquisition vastly different measurement procedures, like FLIM, lifetime based FRET, FCS, or even coincidence correlation ("antibunching") can be performed, based on just one fundamental data format. The TTTR format ist supported by all available TCSPC electronics from PicoQuant. Different modules with up to eight independent TCSPC channels for fast and parallel detection are available which provide highest data throughput, multi-stop possibility, and low dead time to realize short acquisition times.
Single photon sensitive detection for highest sensitivity
 To ensure optimal experimental conditions, four different detector types are available for the LSM upgrade kit:
To ensure optimal experimental conditions, four different detector types are available for the LSM upgrade kit:
- Hybrid PMT, the best allrounder for FLIM, FCS, and NDD deep tissue imaging
- PDM SPAD, the fastest detector for FLIM and FCS
- SPCM-AQRH SPAD, the highest sensitivity detector best for FCS
- Photomultiplier tubes (PMTs) of the PMA series, the economic variant for FLIM
Hybrid PMTs and SPADs offer highest sensitivity as needed for single molecule experiments and are suited for, e.g., cellular FLIM and FCS studies at low fluorescence intensities. The detectors differ in their efficiency, temporal resolution, spectral range, or active area. Thus, the choice of the detector depends on several factors such as the targeted application or interface to the microscope for confocal or NDD (non-descanned) detection.
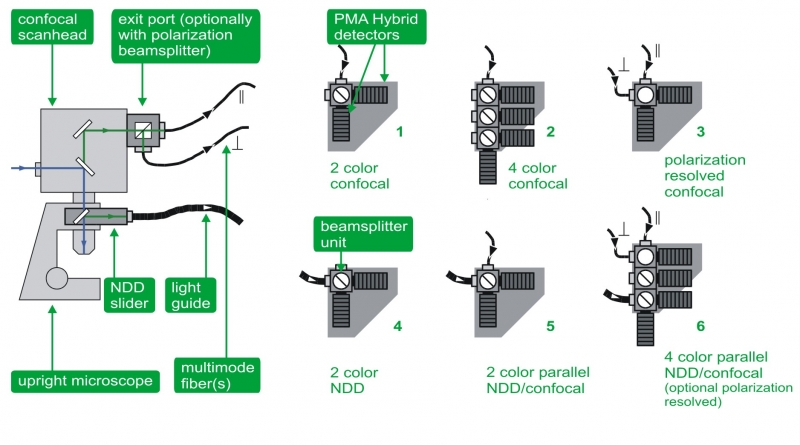
Confocal and NDD detection
The detectors can be attached in confocal mode using a pinhole and fitting to all detector types, as well as in non-descanned detection (NDD) for multi photon excitation.
In the standard confocal mode, the detectors are connected via an optical fiber to the fiber exit port of the microscope. In this confocal configuration set-ups with up to 4 detectors are available. Integrated filter holders allow quick change of emission filters in order to adapt to different experimental conditions and fluorophores.
The non-descanned configuration (NDD) is used in systems with a multi-photon laser and enables deep-tissue FLIM. In this case one or two detectors (PMTs or Hybrid PMTs) are connected to the LSM via a large core liquid light guide. The light guide is either mounted to a suited NDD port of the microscope or, alternatively, a specially developed dove-tail adapter collects the fluorescence directly above the objective. The latter approach features a very high collection efficiency due the light collection very near to objective and the high fluorescence transmission through the liquid light guide. In case of the Olympus FluoView FV1000/1200 MPE system it is also possible to use up to four internal NDD standard PMT detectors of the LSM for FLIM measurements.
Multichannel detection unit
To offer greatest flexibility and multiple applications in one system, the multichannel detection unit allows for parallel confocal, polarization, and NDD measurements with up to four detection channels for multi color FLIM, FRET, deep tissue FLIM imaging, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS) as well as anisotropy studies. Several detection configurations are available:
- Dual-channel confocal detection for FLIM, FRET, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS)
- Four-channel standard confocal detection for FLIM, FRET, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS)
- Dual channel polarization resolved confocal detection for anisotropy imaging
- Dual channel NDD set-up for deep tissue FLIM and FRET
- Parallel dual channel set-up enabling confocal and non-descanned detection for dual color FLIM, FRET, deep tissue FLIM imaging, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS)
- Parallel four channel set-up enabling confocal as well as non-descanned detection and optionally polarization measurements for multi color FLIM, FRET, deep tissue FLIM imaging, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS), and anisotropy.
All options are also available for inverse microscope setups.
more...
Intuitive software
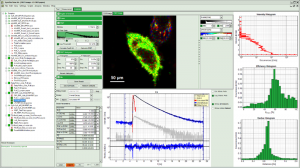 The system software SymPhoTime 64 provides multiple, easy-to-use acquisition and analysis tools for all kind of samples. Based on the sophisticated data collection and handling, the system software SymPhoTime 64 supports a multitude of methods, such as Fluorescence Lifetime Imaging (FLIM), Förster Resonance Energy Transfer (FRET), Fluorescence Correlation Spectroscopy (FCS), Fluorescence Lifetime Correlation Spectroscopy (FLCS), intensity time trace, burst analysis, lifetime histogramming, and anisotropy, to name only a few.
The system software SymPhoTime 64 provides multiple, easy-to-use acquisition and analysis tools for all kind of samples. Based on the sophisticated data collection and handling, the system software SymPhoTime 64 supports a multitude of methods, such as Fluorescence Lifetime Imaging (FLIM), Förster Resonance Energy Transfer (FRET), Fluorescence Correlation Spectroscopy (FCS), Fluorescence Lifetime Correlation Spectroscopy (FLCS), intensity time trace, burst analysis, lifetime histogramming, and anisotropy, to name only a few.
SymPhoTime 64 data handling maintains a transparent data structure where all derived data is stored in one workspace, including a log file to keep track of all measurement and analysis steps. Image data can be processed further or exported to standard formats. A large number of algorithms for those methods are already integrated in the software, providing a analysis platform for ready-to-publish data. At the same time, SymPhoTime 64 offers enhanced flexibility for the integration of novel, cutting edge algorithms by the user. A dedicated scripting language interface allows to modify and expand the analysis routines according to the experimental needs.
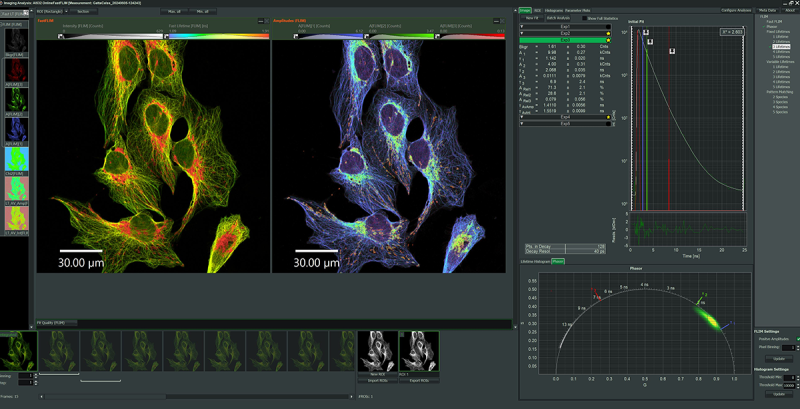 Advanced offline image analysis is possible with the NovaFLIM software package. NovaFLIM features an extremely fast GPU-accelerated analysis of FLIM, FLIM-FRET, and anisotropy data. Efficient batch analysis of z-stacks, time-lapse series and stitched images as well as advanced, flexible and reproducibl ROI handling enables high quality image analysis.
Advanced offline image analysis is possible with the NovaFLIM software package. NovaFLIM features an extremely fast GPU-accelerated analysis of FLIM, FLIM-FRET, and anisotropy data. Efficient batch analysis of z-stacks, time-lapse series and stitched images as well as advanced, flexible and reproducibl ROI handling enables high quality image analysis.
A specially developed interface between the SymPhoTime 64 and the LSM software (Nikon and Leica) strongly facilitates data acquisition and enables straightforward recording of, e.g., FLIM images up to 4096 × 4096 pixels, time series FLIM, z-stacks as well as single and multipoint measurements for FCS. Online previews of the main parameters (Fast FLIM, FCS, time-traces, or TCSPC histograms) allow to quickly optimize data acquisition.
Examples and tutorials
Introducing the rapidFLIM approach
A collection of rapidFLIM applications
Zeiss LSM 880 with PicoQuant FLIM & FCS Upgrade Kit
FLIM and FCS Upgrade for Laser Scanning Microscopes (1/3)
FLIM and FCS Upgrade for Laser Scanning Microscopes (2/3)
FLIM and FCS Upgrade for Laser Scanning Microscopes (3/3)
3D FLIM z-stack of Daisy pollen
Measure an Instrument Response Function (IRF) with a LSM Upgrade Kit
Performing a FCS measurement with a LSM upgrade kit
Measuring a Fluorescence Lifetime Image (FLIM) with a LSM Upgrade Kit
Recording a Fluorescence Lifetime Image (FLIM) Stack with a LSM Upgrade Kit on a Nikon A1
Performing a FLIM-FRET Measurement with a LSM Upgrade Kit
Supported LSMs

AX
A1
C1si, C1
C2+, C2

FluoView FV4000
FluoView FV3000
FVMPE-RS
FluoView FV1200 (MPE)
FluoView FV1000 (MPE)

HyperScope
VivoScope

LSM 980
LSM 880
LSM 780
LSM 710
| Excitation System |
|
| Supported LSMs |
|
| Detection |
|
| Detectors |
|
| Data acquisition |
|
| Software |
|
All Information given here is reliable to our best knowledge. However, no responsibility is assumed for possible inaccuracies or omissions. Specifications and external appearances are subject to change without notice.

- Excitation wavelengths: 375 nm - 900 nm
- Parallel attachment of pulsed lasers from PicoQuant and MPE laser (Nikon A1)
- Simultaneous usage of pulsed lasers from PicoQuant and CW lasers from Nikon A1
- Special fiber switch for incoupling of the pulsed lasers (Nikon C series)
- Attachment of PicoQuant detectors to a special exit port of the LSM (Nikon A1)
- Alternative coupling of PicoQuant detectors and Nikon´s spectral detection unit (Nikon C1si, C2) or Nikon´s PMT detection unit (Nikon C1, C2)
- Parallel setup for confocal and NDD imaging as well as polarization measurements (Nikon A1)
- SymPhoTime 64 integration into the LSM NIS software to facilitate FLIM and FCS data acquisition
- Data analysis using the powerful SymPhoTime 64 software
- Full control of PicoQuant pulsed laser in NIS elements to enable high content FLIM screening using JOBS
For details, please check the selection chart for Nikon A1, C1si and C2+, C1 and C2.

- Excitation wavelengths: 405 - 900 nm (FV3000) and 375 - 900 nm (FV1200 / FV1000)
- Simultaneous usage of pulsed lasers from PicoQuant and CW lasers from Evident, independent of excitation wavelength
- Usage of pulsed lasers with normal or SIM scanner
- Attachment of PicoQuant detectors to a special exit port of the LSM
- Parallel setup for confocal and NDD imaging (FV1000MPE, FV1200MPE) as well as polarization measurements
- Data analysis using the powerful SymPhoTime 64 software
- Usage of up to four internal NDD PMTs for deep-tissue FLIM (FV1000MPE, FV1200MPE)
For details, please check the selection chart for FluoView FV4000, FluoView FV3000, FluoView FV1000 (MPE) / FV1200 (MPE), and FVMPE-RS.

- Usage of MPE laser
- Usage of up to four internal NDD detectors for deep-tissue FLIM
- Usage of PicoQuant single photon detectors for standard NDD imaging
- Parallel set-up for FLIM and intensity based NDD imaging
- SymPhoTime 64 integration into the ScanImage software from VidrioTechnologies to facilitate FLIM data acquisition
- Data analysis using the powerful SymPhoTime 64 software

- Excitation wavelengths: 405 nm - 640 nm
- Parallel attachment of pulsed lasers from PicoQuant and Zeiss
- Parallel attachment of pulsed VIS lasers from PicoQuant and MPE laser
- Attachment of PicoQuant detectors to a special exit port of the LSM
- Simultaneous coupling of PicoQuant detectors and the ConfoCor Unit (LSM 710)
- NDD in combination with NLO for upright and inverse microscopes
- Single as well as dual channel NDD (LSM 710 NLO / LSM 780 NLO/ LSM 980 NLO)
- Parallel setup for confocal and NDD imaging as well as polarization measurements
- Data analysis using the powerful SymPhoTime 64 software
- SymPhoTime 64 integration into the LSM ZEN software to facilitate FLIM and FCS data acquisition
For details, please check the selection chart for Zeiss LSM980, Zeiss LSM 710/780/880/980.

- Excitation wavelengths: 405 nm, 440 nm, 470 nm and 640 nm (TCS SP5 / SP8)
- Simultaneous usage of pulsed lasers from PicoQuant and CW lasers from Leica
- Parallel attachment of the pulsed 405 nm laser from PicoQuant and MPE laser
Intracellular Ca2+ signalling upon mechanical stimulation
Intracellular Ca2+ signals induced by mechanical stimulation of HEK-cells loaded with Oregon-Green-Bapta-1. In this video, the cells were imaged before, during, and after mechincal stimulation of the top two cells. The mechanical stimulation leads to an uptake of calcium ions in the cells. This increase in Ca2+ concentration results in a change of the fluorescence lifetime of OGB-1 AM, and the top two cells light up in green for a short time. The rapidFLIMHiRes method enabled imaging a sample area of 128 x 128 pixels with a speed of 5 FLIM frames per second, which makes it possible to quantitatively observe the rise of calcium ion concentration in the cells over a short time period.
Sample details:
- Human Embryonic Kidney (HEK) cells loaded with Oregon-Green-Bapta-1 (OGB-1 AM) kept in artificial cerebrospinal fluid
Set-up:
- 128 x 128 pixel, 3.81 µs pixel dwell time
- 5 frames per second
Sample and data courtesy of Prof. Rose (Institute of Neurobiology, Heinrich Heine University Düsseldorf, Germany)
Monitoring changes in membrane tension with rapidFLIMHiRes
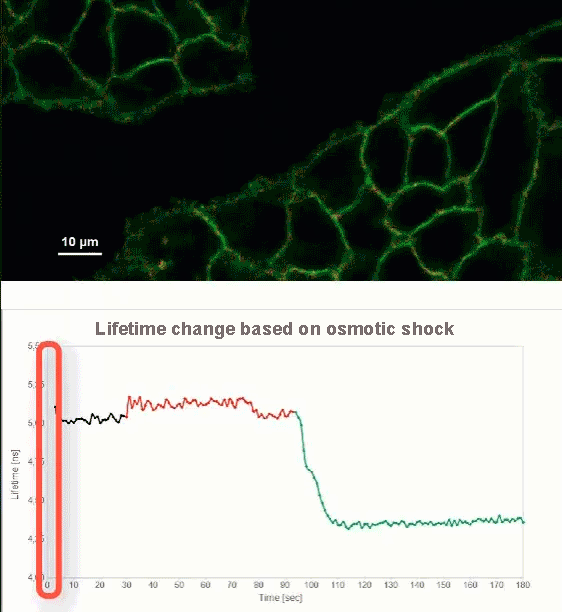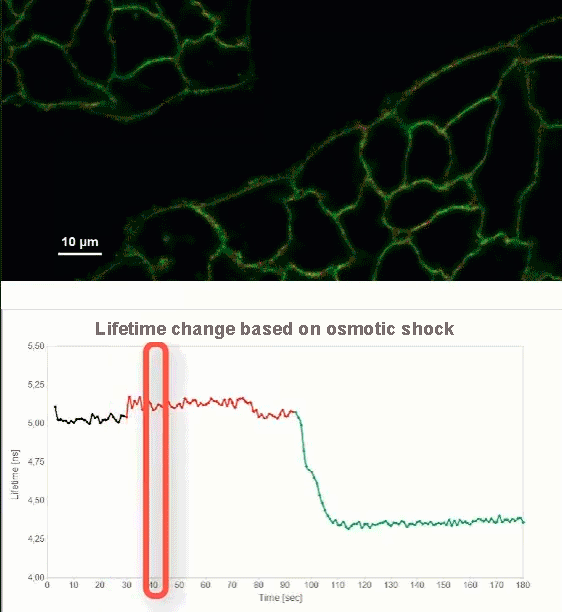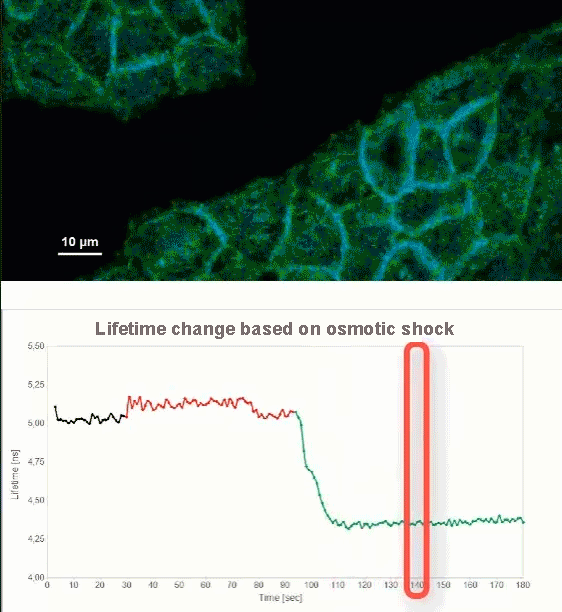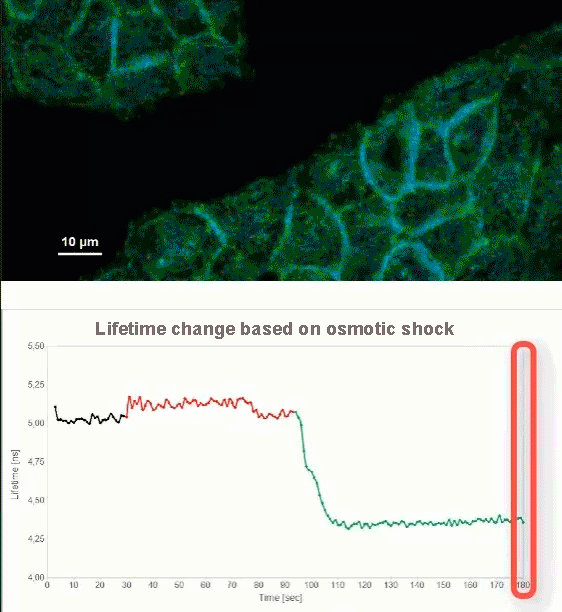
This video shows MDCK cells whose membranes were stained with the fluorescent probe Fliper-TR® (Fluorescent lipid tensor Reporter). This probe displays a significant change in fluorescence lifetime when embedded in a confining environment. If lateral pressure is applied, Fliper-TR® will undergo a planarization which leads to a change in fluorescence lifetime, which allows imaging lipid composition and membrane tension in both live cell and artificial membranes.
Under isoosmotic conditions, Fliper-TR® exhibits a fluorescence lifetime of ca. 5.1 ns. After inducing an hyperosmotic shock, the membrane tension decreases rapidly and a shorter lifetime is observed. Imaging with the rapidFLIMHiRes approach is fast enough so that this lifetime change and by extension the change in membrane tension can be recorded in real time.
Sample details:
- Madin-Darby Canine Kidney (MDCK) cells, membranes stained with Fliper-TR®
Set-up:
- Excitation: 485 nm
- Image size: 512 x 256 pixels
- 1.0 frames per second
Sample courtesy of A. Colom, University of Geneva, Switzerland
Reference: Colom, A., Derivery, E., Soleimanpour, S. et al. A fluorescent membrane tension probe. Nature Chem 10, 1118–1125 (2018). https://doi.org/10.1038/s41557-018-0127-3
rapidFLIM - Redefining standards for dynamic FLIM imaging
rapidFLIM measurements enable the imaging of dynamics in fluorescence lifetime. This new approach allows for fast FLIM acquisition with several frames per second as well as imaging of dynamic processes (e.g., protein interaction, chemical reaction, or ion flux), highly mobile species (e.g., mobility of cell organelles or particles, cell migration), and investigating FRET dynamics. More than 10 frames per second can be acquired, depending on sample brightness and image size.
Unilamellar vesicles can be produced in a size regime ranging from giant (GUVs) to large (LUVs), and even to small (SUVs). The flexibility in membrane composition and possibility to introduce specifically labeled lipids increase the importance of unilamellar vesicles for studies in cell biology. Thus turning such vesicles a very powerful model to investigate e.g., membrane domain formation as well as lipid organization in membrane micro-domains.
Until now, acquiring FLIM images took up to several minutes and due to the high mobility of GUV's, imaging them accurately is difficult. Applying the rapidFLIM approach significantly decreases the acquisition time, allowing to record up to several frames per seconds. Thus even highly mobile GUVs can be precisely tracked. In this example, two fluorophore labeled lipids (C6-NBD-PC and N-Rhd-DOPE) were incorporated into GUVs. In non-phase separated GUVs, the lifetime of NBD (7-nitrobenz-2-oxa-1,3-diazol-4-yl) is strongly quenched (down to ~2 ns) due to FRET to the acceptor rhodamine. The video shown here contains 300 frames that were recorded with a frame rate of 5.6 fps.
Sample details:
- GUVs with NBD and Rhodamin labeled lipids (no phase separation): DOPC + 0.5 mol % Palmitoyl-C6-NBD-PC + 0.5 mol % N-Rhd-DOPE
- NBD fused to Palmitoyl-C6-NBD-PC (phosphatidyl choline)
- Rhodamine fused to N-Rhd-DOPE (Di-oleyl-phosphatidyl-ethanolamin)
Set-up:
- Excitation: 485 nm, 40 MHz
- Long pass filter: 488 nm
- 75 x 75 µm, 300 x 300 pixel, 1 µs/pixel
- 300 frames with 5.6 fps
GUV preparation by Ivan Haralampiev, Lab of Molecular Biophysics, Humboldt Universität zu Berlin
Lipid Order Determination Using Fluorescence Lifetime
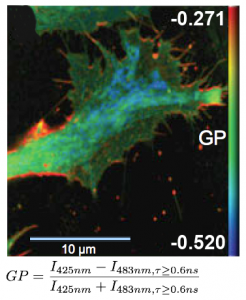 FLIM measurements facilitate the differentiation between ordered and disordered membrane phases. The membrane dyes Laurdan and di-4-ANEPPDHQ can be used to image membrane order due to a spectral blue-shift in the fluorescence emission as well as a lifetime shift between the liquid-ordered and liquid-disordered phases. These images typically take the form of a normalized intensity ratio image known as a generalized polarization (GP) plot. Here, the known excited state photo physics is exploited via Time-Correlated Single-Photon Counting (TCSPC) to demonstrate GP contrast enhancement for these two probes. The image shows a GP plot of a Laurdan stained, fixed BAEC cell that combines lifetime and spectral changes. The plasma membrane at the cell surface shows higher order (red) compared to the inner cell compartments (blue).
FLIM measurements facilitate the differentiation between ordered and disordered membrane phases. The membrane dyes Laurdan and di-4-ANEPPDHQ can be used to image membrane order due to a spectral blue-shift in the fluorescence emission as well as a lifetime shift between the liquid-ordered and liquid-disordered phases. These images typically take the form of a normalized intensity ratio image known as a generalized polarization (GP) plot. Here, the known excited state photo physics is exploited via Time-Correlated Single-Photon Counting (TCSPC) to demonstrate GP contrast enhancement for these two probes. The image shows a GP plot of a Laurdan stained, fixed BAEC cell that combines lifetime and spectral changes. The plasma membrane at the cell surface shows higher order (red) compared to the inner cell compartments (blue).
Set-up:
- Combined MicroTime 200 and LSM Upgrade Kit (Leica TCS SP5)
- Excitation: two-photon excitation at 800 nm, SpectraPhysics MaiTai
- Analysis: SymPhoTime
Courtesy of Katharina Gaus, Centre for Vascular Research, University of New South Wales, Sydney, Australia
Reference: Owen et al., Microsc. Res. Tech. 73(6), (2010)
The compact FLIM and FCS upgrade kit enables multiple time-resolved applications with Laser Scanning Microscopes (LSMs), such as:
- Time-Resolved Fluorescence
- NEW – rapidFLIMHiRes - Redefining standards for dynamic FLIM imaging
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Fluorescence Cross-Correlation Spectroscopy (FCCS)
- Foerster Resonance Energy Transfer (FRET)
- Pulsed Interleaved Excitation (PIE)
- Laser Cutting/Ablation
- Pattern Matching Analysis
- Time-Resolved Photoluminescence (TRPL)
- TRPL Imaging
- Antibunching
- Anisotropy
The following documents are available for download:
- Brochure LSM Upgrade Kit
- Selection chart for Nikon AX
- Selection chart for Nikon A1
- Selection chart for Nikon C1
- Selection chart for Nikon C1si and C2 with Lu4 Laser Combiner
- Selection chart for Nikon C2+ with LUN Laser Combiner
- Selection chart for Olympus FV1000 and FV1200
- Selection chart for Olympus FV3000
- Selection chart for Olympus FVMPE-RS
- Selection chart for Zeiss LSM 710/780/880/980
- NIS-Elements PicoQuant FLIM & FCS Software Control Plug-in
- Poster: Quick Reference for confocal time-resolved microscopy (FLIM, FRET, FCS)
- Practical Manual for Fluorescence Microscopy Techniques
Presentations (as PDF files)
Related technical and application notes:
- Technical note: Combination of pulsed and cw lasers on a Olympus LSM
- Technical note: FLIM NDD Upgrade Kit for the Olympus FluoView FV1000MPE
- Technical note: Polarization Extension Unit for LSM Upgrade Kits
- Technical note: ROI scanning using pulsed lasers on a Olympus FluoView
- Technical note: Time-Correlated Single Photon Counting (TCSPC)
- Application note: Fluorescence Lifetime Imaging (FLIM) in Confocal Microscopy
- Application note: Imaging of molecular distances using FLIM-FRET
- Application note: FRET analysis with Pulsed-Interleaved Excitation (PIE)
- Application note: Time-gated Fluorescence Correlation Spectoscopy
- Application note: Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Technical note: Phosphorescence Lifetime Imaging Microscopy (PLIM) Measurements - Practical Aspects
- Application note: Visualize dynamic processes with rapidFLIMHiRes, the ultra fast FLIM imaging method with outstanding time resolution
Latest 10 publications referencing LSM Upgrade Kit, SymPhoTime
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this product in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.




 Contact us
Contact us