-
Applications
-
Life Science
- rapidFLIMHiRes (Fluorescence Lifetime Imaging) New
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Foerster Resonance Energy Transfer (FRET)
- Pulsed Interleaved Excitation (PIE)
- scanning Fluorescence Correlation Spectroscopy (sFCS) New
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Dual-focus Fluorescence Correlation Spectroscopy (2fFCS)
- Stimulated Emission Depletion Microscopy (STED)
- Single Molecule Detection
- Time-resolved Fluorescence
- Fluorescence Anisotropy (Polarization)
- Pattern Matching Analysis
- Two-Photon Excitation (TPE)
- Diffuse Optical Tomography and Imaging
- Singlet Oxygen
- Laser Cutting/Ablation
- Materials Science
- Metrology
- Quantum Optics
-
Life Science
Life Science
Phosphorescence Lifetime Imaging (PLIM)
A time-resolved Imaging technique based on long lived excited states in luminescent materials
Phosphorescence Lifetime Imaging (PLIM) is similar to Fluorescence Lifetime Imaging (FLIM), only that it images the phosphorescence from the sample and consequently covers time ranges up to milliseconds. Analogous to FLIM, the contrast in a PLIM image is based on the lifetime of individual fluorophores rather than their emission spectra. The phosphorescence lifetime is defined as the average time that a molecule remains in an excited state prior to returning to the ground state by emitting a photon.
PLIM, or generally the characterization of phosphorescent compounds has been of great importance in the field of materials science namely chemical sensing for many years , and has renewed its interest over the past decade with the booming development of Organic Light Emitting Diode (OLED) technology. Other typical samples include metal ions complexed with organic ligands, which can be used to, e.g., image oxygen consumption in living cells.
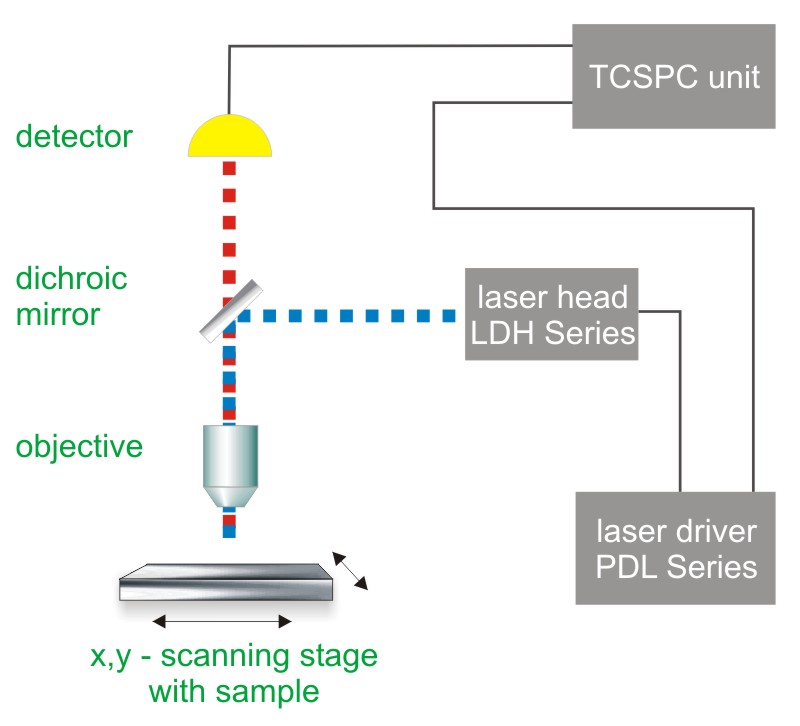
 Multi-channel scaling (MCS) or Time-Correlated Single Photon Counting (TCSPC) with multi-stop capabilities is used to determine the phosphorescence lifetime. In both cases, one measures the time between sample excitation by a pulsed laser and the arrival of the emitted photon at the detector. The methods requires a defined “start”, provided by the electronics steering the laser pulse or a photodiode, and a defined “stop” signal, realized by detection with single-photon sensitive detectors (e.g. Single Photon Avalanche Diodes, SPADs or Photomultiplier Tubes, PMTs). The measurement of this time delay is repeated many times to account for the statistical nature of the fluorophores emission. The delay times are sorted into a histogram that plots the occurrence of emission over time after the excitation pulse.
Multi-channel scaling (MCS) or Time-Correlated Single Photon Counting (TCSPC) with multi-stop capabilities is used to determine the phosphorescence lifetime. In both cases, one measures the time between sample excitation by a pulsed laser and the arrival of the emitted photon at the detector. The methods requires a defined “start”, provided by the electronics steering the laser pulse or a photodiode, and a defined “stop” signal, realized by detection with single-photon sensitive detectors (e.g. Single Photon Avalanche Diodes, SPADs or Photomultiplier Tubes, PMTs). The measurement of this time delay is repeated many times to account for the statistical nature of the fluorophores emission. The delay times are sorted into a histogram that plots the occurrence of emission over time after the excitation pulse.
In order to acquire a phosphorescence lifetime image, the photons have to be attributed to the different pixels, which is done by storing the absolute arrival times of the photons additionally to the relative arrival time in respect to the laser pulse. Line and frame marker signals from the scanner of the confocal microscope are additionally recorded in order to sort the time stream of photons into the different pixels.
In contrast to TCSPC based FLIM, PLIM can usually be performed efficiently with galvo scanners for compounds with lifetimes up to a few µs only due to their high scanning speeds that limit the dwell time per pixel. If the dwell time is shorter than the time needed for the phosphorescence to decay completely, photons will be sorted into wrong pixels, leading to distorted images. PLIM is therefore best performed with piezo scanning devices, which enable lower scanning speeds than galvo scanners. As the ideal pixel dwell time is ~50-100 times the luminescence lifetime, measurements on dyes with slow phosphorescence can take a very long time.
Consequently the essential components of a FLIM set-up are:
- pulsed laser source - for components with lifetimes >1µs, excitation using pulse trains is recommended in order to increase the excitation efficiency
- single photon sensitive detector
- dichroic mirror (to separate fluorescence signal from excitation light)
- objective (to focus the excitation light into the sample and collect fluorescence signal)
- MCS or TCSPC unit with multi-stop capabilities to measure the time between excitation and fluorescence emission
Related technical and application notes:
The following PicoQuant systems provide access to PLIM experiments and other lifetime measurements
Single Photon Counting Confocal Microscope
Luminosa combines state-of-the-art hardware with cutting edge software to deliver highest quality data while simplifying daily operation. The software includes context-based workflows for FLIM, FRET, and FCS, which allow the users to focus on their samples. Tight integration of hardware and software enable automated routines, e.g., for auto-alignment or laser power calibration, which increase ease of use as well as reproducibility of experiments. Still, an open mode of operation is available for full access to every optomechanical component via software.
Modular, time-resolved confocal fluorescence microscopy platform
The MicroTime 200 time-resolved fluorescence microscope system is a powerful instrument capable of Fluorecence Corelation Spectroscopy and its daughter techniques as well as Fluorescence Lifetime Imaging (FLIM) and Phosphorescence Liftetime Imaging (PLIM) with single molecule detection sensitivity. It contains the complete optics and electronics for recording virtually all aspects of the fluorescence dynamics of microscopic samples or femtoliter volumes. The instrument gains its exceptional sensitivity and flexibility in combination with unprecedented ease-of-use from a unique fusion of miniaturized and highly sophisticated state-of-the-art technologies. Although, these technologies enable to run an instrument of comparable complexity and power without having to spend more time on instrument maintenance than on original scientific content, the MicroTime200 remains an open platform that allows the advanced scientist to easily built upon the open character of the instrument in order to realize highly customized applications
LSM Upgrade Kits from PicoQuant add time-resolved capabilities to laser scanning microscopes from many major manufactures
Confocal Laser Scanning Microscopes (LSMs) are widely used tools in cell and molecular biology, biochemistry and other related sciences. PicoQuant's LSM Upgrade Kits greatly enhance the capabilities of these microscopes by extension to time-resolved techniques, and thereby providing not only Fluorescence Lifetime Imaging (FLIM) and Phosphorescence Liftetime Imaging (PLIM) but also Fluorescence Correlation Spectroscopy (FCS) and a wealth of other time resolved techniques. The LSM Upgrade Kit combines PicoQuant products to a ready-to-use kit that fits your specific application on a state of the art Laser Scanning Microscope of your choice from Leica, Nikon, Olympus or Zeiss.
Upright time-resolved confocal microscope
The upright time-resolved microscope MicroTime 100 contains the complete optics and electronics for recording F(C)CS or FLCS data as well as fluorescence decays in small volumes by means of Time-Correlated Single Photon Counting (TCSPC). The system is based on a conventional upright microscope body that permist easy access to a wide range of sample shapes and sizes.
 The following core components are needed to build a system capable of PLIM measurements, which are (partly) available from PicoQuant:
The following core components are needed to build a system capable of PLIM measurements, which are (partly) available from PicoQuant:
- Laser drivers
- Laser or LED heads
- Photon Counting Detector
- TCSPC Electronics
- Analysis software
- Scanning device
- Piezo-Scanner
- Confocal optics
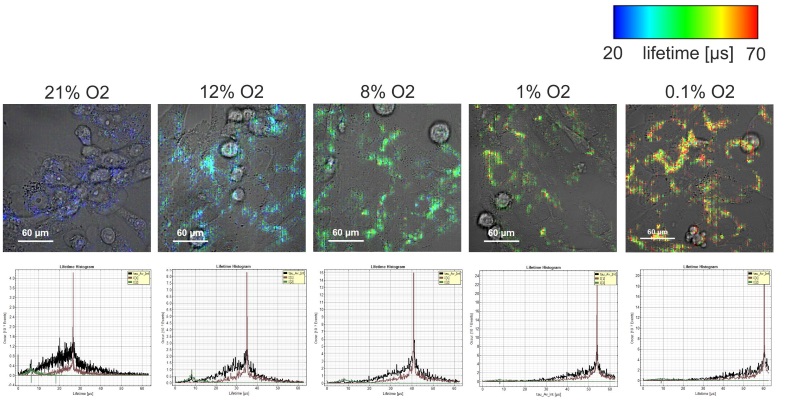
Using phosphorescence lifetime imaging (PLIM) to measure oxygen tension in live cells
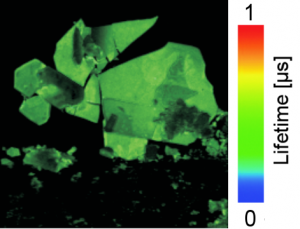
The homeostasis of oxygen plays a major role for the maintenance of cellular health and function. Oxygen deprivation, also called hypoxia has been identified as one of the main contributors to tumour aggressiveness. In order to study how hypoxia influences cancer cell behaviour the understanding of its oxygenation is crucial. Here we utilise PLIM to measure oxygen tension within living cells. Intracellular oxygen tension was measured in human neuroblastoma (SK-N-AS) cells based on the quenched phosphorescence detection of Pt-porphyrin based nanoparticles. Cells were incubated with the self-permeable, self-loading nanoparticle NanO2 (Luxcel Biosciences or Ibidi) according to manufacturer’s instructions and imaged using a Zeiss LSM780 with Picoquant Timeharp 260. PLIM measurements were performed at decreasing steps of extracellular oxygen tensions (from 21 % to 0.1 % O2). Cells were left to adjust to the new external oxygen tension for at least 30 mins prior lifetime measurements. Lifetime was found to increase (30, 33.8, 40.9, 53.9 and 60 µs) with decreasing extracellular oxygen 21, 12, 8, 1 and 0.1 %. Calibration was performed with a respiration inhibitor, Antimycin A and at 21 % O2 allowing the conversion of phosphorescence lifetime signal into intracellular O2 concentration [µM].
Set-up:
- Zeiss 780 with LSM Upgrade Kit
- TCSPC electronics: TimeHarp 260 and LongRange mode
- Excitation: 440 nm
- Emission: 570 nm - 694 nm
- Objective: 40x/1.20 W
- Detection: PMA Hybrid
- Sequencer period 14184 pulses, i.e. 177 µs = 5.6 kHz
- Frame size: 256 x 256
- Data processing: SymPhoTime 64
Data courtesy of Violaine See, Centre for Cell Imaging, Institute of Integrative Biology, Liverpool, UK
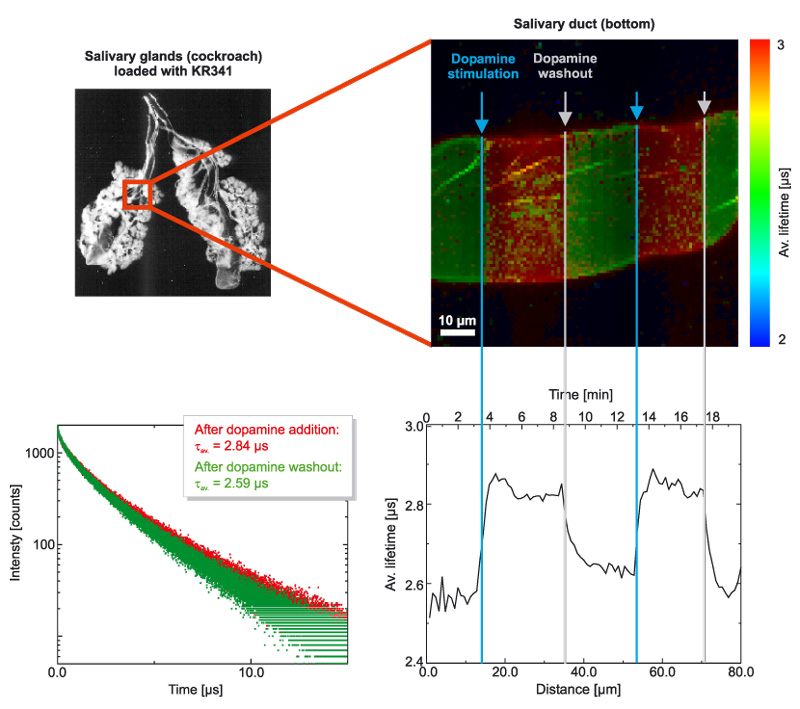
Phosphorescent probe allows imaging O2 concentrations in salivary glands
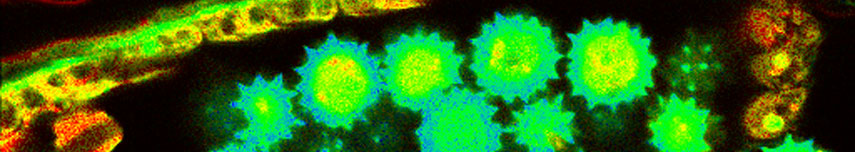
Oxygen was imaged in male cockroach salivary glands that were incubated with KR341 (a phosphorescent probe on Ru-basis with a lifetime in the µs-range that is quenched by molecular oxygen). The sample was slowly scanned and during the image scan, dopamine was added or removed by washing with Ringer buffer. Stimulation of cellular metabolism by dopamine exposure decreases the oxygen concentration within the salivary glands, which can be measured by an increase in the phosphorescence lifetime of KR341.
Set-up:
- MicroTime 200 with TimeHarp 260
- Excitation: 470 nm, 50 kHz repetition rate
- Emission: > 500 nm
- Pixel dwell time 25 ms/picel, image size: 80 x 80 µm
- Data processing: SymPhoTime
Data courtesy of K. Jahn, C. Hille, University of Potsdam, Germany
KR341 was kindly provided by Rolf Kramer, University of Freiburg, Germany
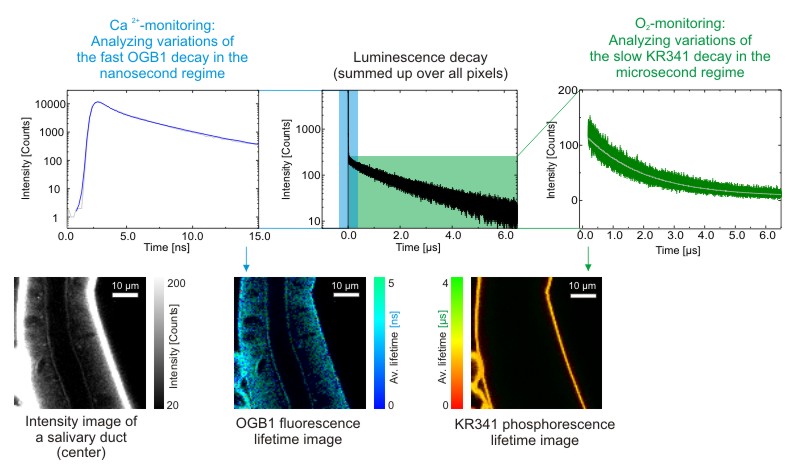
Combining FLIM and PLIM for simultaneous monitoring of Ca2+ and O2 by using multiple luminescent probes
Cockroach salivary glands were labeled with KR341 (a phosphorescent probe on Ru-basis with a lifetime in the µs-range, that is quenched by molecular oxygen) and OGB1 (a fluorescent, calcium-sensitive probe with a lifetime in the ns-range). As the lifetimes of the two labels are very different, they can be separated and analyzed independently. The result are separate FLIM and PLIM images, from which the O2 and Ca2+-concentrations can be derived by analyzing local changes in the lifetime.
Set-up:
- MicroTime 200 with TimeHarp 260
- Excitation: 470 nm, 50 kHz repetition rate
- Emission: > 500 nm
- Pixel dwell time 25 ms/picel, image size: 80 x 80 µm
- Data processing: SymPhoTime
Data courtesy of K. Jahn, C. Hille, University of Potsdam, Germany
KR341 was kindly provided by Rolf Kramer, University of Freiburg, Germany
Latest 10 publications related to FLIM
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this application and our products in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.

 Contact us
Contact us Luminosa
Luminosa MicroTime 200
MicroTime 200 LSM Upgrade Kit
LSM Upgrade Kit MicroTime 100
MicroTime 100