Materials Science
Photocatalysis
Singlet Oxygen Detection, CO2 Photoreduction, H2 Production, and Environmental Purification
Photocatalysis is a process that utilizes the energy of light to activate a substance, known as a photocatalyst, to drive chemical reactions. It has a wide range of applications, including environmental purification, and chemical synthesis. The photocatalyst typically is a semiconducting material like titanium dioxide or zinc oxide. When the photocatalyst is exposed to light, electrons in the material are excited from the valence band to the conduction band, creating electron-hole pairs. These charge carriers are essential for driving chemical reactions.
Improving the efficiency and selectivity of photocatalysts is essential to maximize their performance in various applications. Ongoing research in this field pursues various strategies, such as sensitization, enhancing charge separation, or improving light utilization.
- Singlet oxygen detection
- CO2 photoreduction
- Photocatalytic hydrogen generation
- Environmental purification
- Polymerization with upconversion materials
Contact us
Singlet oxygen detection
 Singlet oxygen is a highly reactive form of molecular oxygen that is produced when oxygen absorbs energy and transitions from its ground state to an excited singlet state. Singlet oxygen is generated during various processes, for example by photosensitizers during photodynamic therapy (PDT) for cancer treatment, or during the immune response to pathogens. In biological systems, it leads to oxidative stress.
Singlet oxygen is a highly reactive form of molecular oxygen that is produced when oxygen absorbs energy and transitions from its ground state to an excited singlet state. Singlet oxygen is generated during various processes, for example by photosensitizers during photodynamic therapy (PDT) for cancer treatment, or during the immune response to pathogens. In biological systems, it leads to oxidative stress.
Luminescence microscopy and spectroscopy methods enable researchers to quantify the presence of singlet oxygen, track its spatial and temporal distribution, and understand its role in different processes.
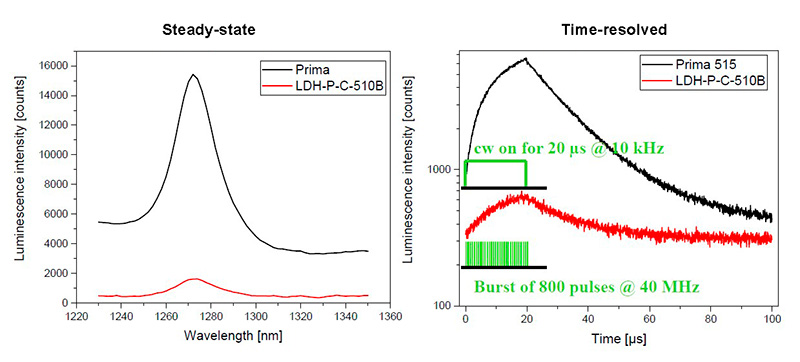 The luminescence signal of singlet oxygen is relatively weak and hard to measure because in the NIR range around 1270 nm current detectors suffer from low sensitivity. Therefore, excitation efficiency is particularly important. LDH laser heads can be operated in burst mode using a PDL 828 laser driver to efficiently excite singlet oxygen. The new Prima laser module offers a fast CW switching mode which proves to be even more effective to enhance signal. The time-resolved fluorescence spectrometer FluoTime 300 is applied for emission spectra and decay acquisition.
The luminescence signal of singlet oxygen is relatively weak and hard to measure because in the NIR range around 1270 nm current detectors suffer from low sensitivity. Therefore, excitation efficiency is particularly important. LDH laser heads can be operated in burst mode using a PDL 828 laser driver to efficiently excite singlet oxygen. The new Prima laser module offers a fast CW switching mode which proves to be even more effective to enhance signal. The time-resolved fluorescence spectrometer FluoTime 300 is applied for emission spectra and decay acquisition.
Download our poster to learn more: Photoluminescence studies with Prima
Singlet oxygen generated by Tetraphenylporphyrin (H2TTP)
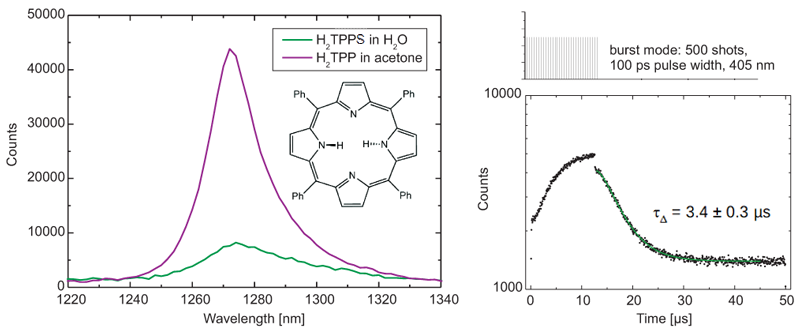 Steady-state and time-resolved emission spectra of singlet oxygen produced by the photosensitizer H2TTP in water and acetone were acquired with a FluoTime 300 time-resolved spectrometer, equipped with a NIR detector and a pulsed 405 nm excitation laser with burst mode.
Steady-state and time-resolved emission spectra of singlet oxygen produced by the photosensitizer H2TTP in water and acetone were acquired with a FluoTime 300 time-resolved spectrometer, equipped with a NIR detector and a pulsed 405 nm excitation laser with burst mode.
The emission peaks of water and singlet oxygen overlap and furthermore water quenches phosphorescence, which makes these measurements challenging. Still, the phosphorescence lifetime of 3.4 ± 0.3 µs obtained with a tail fit excellently agrees with published literature value of 3.7 µs.
Singlet oxygen generated by Zinc phthalocyanine (ZnPc)
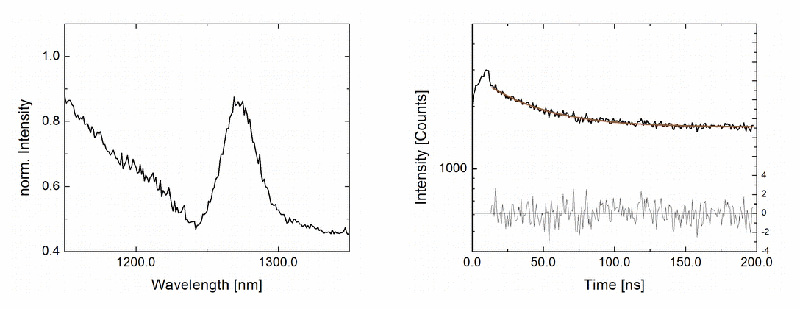 Steady-state emission spectra and phosphorescence decays of singlet oxygen produced by the photosensitizer ZnPc in acetone were acquired with a MicroTime 100 confocal time-resolved microscope fiber-coupled to a FluoTime 300 time-resolved spectrometer, equipped with a NIR detector and a pulsed 660 nm excitation laser using burst mode. The small signal originating from the confined confocal volume was still visible, highlighting the instrument's sensitivity.
Steady-state emission spectra and phosphorescence decays of singlet oxygen produced by the photosensitizer ZnPc in acetone were acquired with a MicroTime 100 confocal time-resolved microscope fiber-coupled to a FluoTime 300 time-resolved spectrometer, equipped with a NIR detector and a pulsed 660 nm excitation laser using burst mode. The small signal originating from the confined confocal volume was still visible, highlighting the instrument's sensitivity.
 PicoQuant solutions to investigate photocatalysis:
PicoQuant solutions to investigate photocatalysis:
- Micro-photoluminescence Upgrade - Microscope-spectrometer coupling >
- FluoTime 300 - High-End Photoluminescence Spectrometer >
- FluoTime 250 - Compact Lifetime Fluorometer >
- MicroTime 100 - Compact Upright Photoluminescence Microscope >
- FluoMic - Compact Upright Widefield Photoluminescence Microscope >
- MicroTime 200 - Confocal Microscopic Platform >
- Wavelength Selection Unit FlexLambda >
CO2 photoreduction
CO2 photoreduction is a process that aims to convert carbon dioxide (CO2) into valuable chemical products, such as hydrocarbons or other fuels. Photocatalysts can absorb light and activate CO2 molecules, leading to the reduction of CO2 into organic compounds.
Electron transfer pathway studied with time-resolved spectroscopy
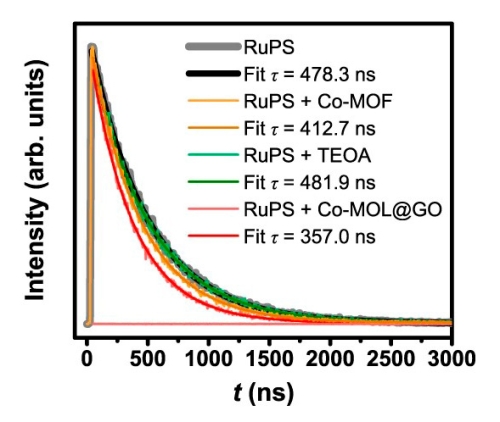 Jia-Wei Wang, Li-Zhen Qiao and colleagues synthesized and characterized a new hybrid catalyst for visible-light-driven CO2 photoreduction with a 34 times higher total CO yield than other state-of-the-art catalysts.
Jia-Wei Wang, Li-Zhen Qiao and colleagues synthesized and characterized a new hybrid catalyst for visible-light-driven CO2 photoreduction with a 34 times higher total CO yield than other state-of-the-art catalysts.
To achieve this, they developed a facile and efficient strategy to construct a heterogeneous catalyst consisting of ultrathin 2D metal-organic layers with graphene oxide as both template and electron mediator. RuPS was used as a photosensitizer.
Among other tests, the researchers studied the electron transfer pathway of the new composite material with time-resolved spectroscopy at 450 nm excitation using a MicroTime 200 time-resolved confocal microscope. The observed shortening of RuPS's fluorescence lifetime confirmed an acceleration of charge transfer to the catalyst, compared to previous bulky catalysts.
Read the paper published in Nature Communications to learn more: Facile electron delivery from graphene template to ultrathin metal-organic layers for boosting CO2 photoreduction

PicoQuant solutions to investigate photocatalysis:
- Micro-photoluminescence Upgrade - Microscope-spectrometer coupling >
- FluoTime 300 - High-End Photoluminescence Spectrometer >
- FluoTime 250 - Compact Lifetime Fluorometer >
- MicroTime 100 - Compact Upright Photoluminescence Microscope >
- FluoMic - Compact Upright Widefield Photoluminescence Microscope >
- MicroTime 200 - Confocal Microscopic Platform >
- Wavelength Selection Unit FlexLambda >
Photocatalytic hydrogen generation
Hydrogen production via photocatalysis involves the use of photocatalysts to split water (H2O) into hydrogen (H2) and oxygen (O2) when exposed to light. Common photocatalysts for hydrogen generation include titanium dioxide and various metal-based catalysts. This process has the potential to be a clean and sustainable method for hydrogen production.
Recombination pathways investigated with TRPL spectroscopy
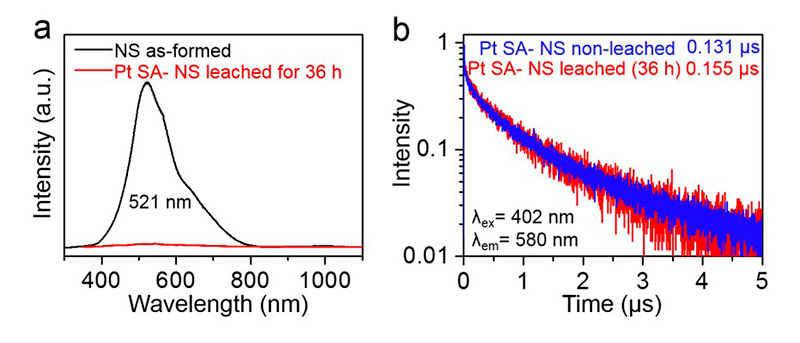 Single platinum (Pt) atoms on titanium dioxide (TiO2) substrate have been reported as a highly efficient co-catalyst in solar hydrogen (H2) production. Noble metal co-catalysts accelerate the charge transfer across the semiconductor interface. Mostly, Pt nanoparticles are decorated onto the TiO2 substrate, but lately, single Pt atoms are increasingly used. Here, researchers aim to identify the truly active form of Pt on TiO2 surface – nanoparticles, Pt0, or positively charged Ptδ+.
Single platinum (Pt) atoms on titanium dioxide (TiO2) substrate have been reported as a highly efficient co-catalyst in solar hydrogen (H2) production. Noble metal co-catalysts accelerate the charge transfer across the semiconductor interface. Mostly, Pt nanoparticles are decorated onto the TiO2 substrate, but lately, single Pt atoms are increasingly used. Here, researchers aim to identify the truly active form of Pt on TiO2 surface – nanoparticles, Pt0, or positively charged Ptδ+.
Electron recombination kinetics were investigated with TRPL spectroscopy using a FluoTime 300 time-resolved fluorescence spectrometer featuring a pulsed 405 nm excitation laser. Decorating the surface with Pt atoms or nanoparticles leads to quenching of the photoluminescence peak at 521 nm, indicating a surface state-related recombination pathway. Removing most of the deposited Pt with cyanide leaching does not significantly affect the PL lifetime decay and photocatalytic activity, showing that only very few hot spots on the surface are truly active.
Read the paper published in RRL Solar to learn more: A Few Pt Single Atoms Are Responsible for the Overall Co-Catalytic Activity in Pt/TiO2 Photocatalytic H2 Generation
 PicoQuant solutions to investigate photocatalysis:
PicoQuant solutions to investigate photocatalysis:
- Micro-photoluminescence Upgrade - Microscope-spectrometer coupling >
- FluoTime 300 - High-End Photoluminescence Spectrometer >
- FluoTime 250 - Compact Lifetime Fluorometer >
- MicroTime 100 - Compact Upright Photoluminescence Microscope >
- FluoMic - Compact Upright Widefield Photoluminescence Microscope >
- MicroTime 200 - Confocal Microscopic Platform >
- Wavelength Selection Unit FlexLambda >
Environmental purification
Photocatalysis is employed to degrade organic dyes, synthetic pigments from the food industry, pesticides, and other pollutants in water and air. The process is highly attractive due to its ability to harness renewable energy sources (solar or artificial light) for environmental cleanup.
Charge recombination efficiency investigated with TRPL spectroscopy
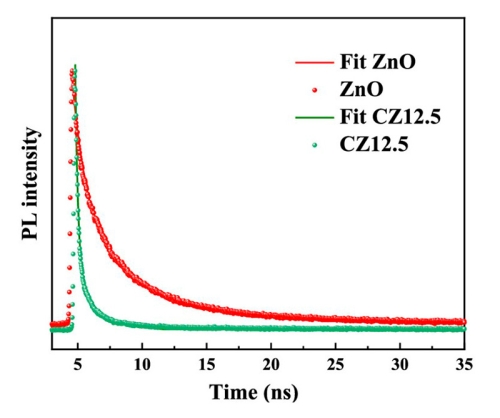 Zinc oxide (ZnO) exhibits a high catalytic activity and electron exchange performance, making it an attractive, environmentally friendly photocatalyst for removal of the synthetic pigment pollutants. But ZnO nanoparticles can only be excited with UV light due to their wide band gap. Here, carbon quantum dots (CQDs) with special up-conversion luminescence were combined with ZnO nanoparticles to construct CQDs/ZnO composites with effectively increased light absorption.
Zinc oxide (ZnO) exhibits a high catalytic activity and electron exchange performance, making it an attractive, environmentally friendly photocatalyst for removal of the synthetic pigment pollutants. But ZnO nanoparticles can only be excited with UV light due to their wide band gap. Here, carbon quantum dots (CQDs) with special up-conversion luminescence were combined with ZnO nanoparticles to construct CQDs/ZnO composites with effectively increased light absorption.
Efficient charge transfer and separation processes between CQDs and ZnO are crucial for the photodegradation reaction. TRPL spectroscopy with a FluoTime 300 time-resolved fluorescence spectrometer equipped with a 405 nm excitation laser was used for investigating the recombination efficiency, where weak PL intensity means low recombination efficiency. The obtained PL decays were fit with a triple-exponential model to account for radiative recombination as well as nonradiative recombination due to trapping and transferring. The shorter lifetime of the composite indicates a slower charge recombination, due to a more effective electron transport.
To learn more, read the paper published in ACS Omega (2023): ZnO Nanoparticles Modified by Carbon Quantum Dots for the Photocatalytic Removal of Synthetic Pigment Pollutants
Interfacial charge-transfer dynamics studied with TRPL spectroscopy
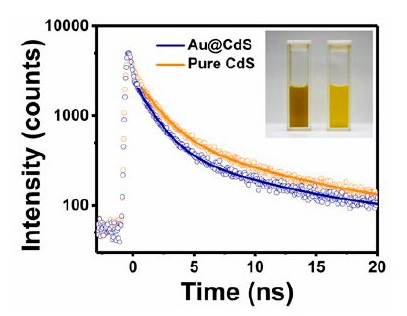 Metal−semiconductor yolk−shell nanocrystals, which consist of Au yolk associated with various semiconductor shells, exhibit significant charge-transfer dynamics between the yolk and shell components. These lead to a charge-carrier separation that can be used for photocatalytical applications, such as dye degradation for environmental purification, hydrogen production, or carbon dioxide reduction.
Metal−semiconductor yolk−shell nanocrystals, which consist of Au yolk associated with various semiconductor shells, exhibit significant charge-transfer dynamics between the yolk and shell components. These lead to a charge-carrier separation that can be used for photocatalytical applications, such as dye degradation for environmental purification, hydrogen production, or carbon dioxide reduction.
Time-resolved photoluminescence (TRPL) spectroscopy was used to gain insight into the interfacial charge transfer. TRPL spectra were measured with a custom-built setup including a sub-nanosecond pulsed LED at 320 nm (PLS 320).
Compared to the pure shell material, all yolk-shell nanocrystals show faster PL decay dynamics, which prove the effective charge separation. To get more quantitative results, the decays were analyzed with a bi-exponential fit model, which showed an additional nonradiative charge transfer and allowed calculation of the charge-transfer rate constant.
To learn more, read the paper published in ACS Appl. Nano Mater. (2022): Electronic Interactions and Charge-Transfer Dynamics for a Series of Yolk–Shell Nanocrystals: Implications for Photocatalysis
 PicoQuant solutions to investigate photocatalysis:
PicoQuant solutions to investigate photocatalysis:
- Micro-photoluminescence Upgrade - Microscope-spectrometer coupling >
- FluoTime 300 - High-End Photoluminescence Spectrometer >
- FluoTime 250 - Compact Lifetime Fluorometer >
- MicroTime 100 - Compact Upright Photoluminescence Microscope >
- FluoMic - Compact Upright Widefield Photoluminescence Microscope >
- MicroTime 200 - Confocal Microscopic Platform >
- Wavelength Selection Unit FlexLambda >
Polymerization with upconversion materials
Luminescence upconversion is a photophysical process in which two or more low-energy photons are absorbed sequentially and converted into a higher-energy photon through a nonlinear process. Ordered inorganic materials, which typically contain rare earth ions, are capable of three types of upconversion: excited state absorption, energy transfer, and photon avalanche. In molecules, triplet-triplet annihilation can additionally take place. Upconversion materials find applications in various fields, for example in photovoltaics for enhanced light harvesting, in bioimaging for in vivo deep tissue imaging, or in security and anti-counterfeiting.
We thank Prof. Nyokong, and Edith Antunes, Rhodes University, South Africa for the NaYF4:Yb/Er nanoparticles with silica coating and Dr. U. Resch-Genger from BAM, Germany for the Yb/Er nanoparticles.
Photoluminescence characterization of NaYF4:Yb/Er upconversion nanoparticles
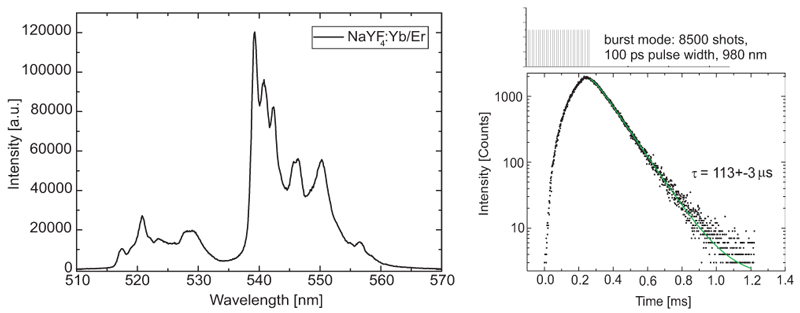 Steady-state and time-resolved luminescence spectra of NaYF4:Yb/Er upconversion nanoparticles in acetonitrile were measured using a FluoTime 300 time-resolved spectrometer, equipped with a 980 nm excitation laser (LDH-D-C-980). The laser was controlled by a PDL 828 driver, which can operate it in either CW, pulsed or burst mode, the latter being the most efficient excitation mode for luminescence decays in the µs range. The luminescence decay was detected at 540 nm. The lifetime value indicates from which electronic state the emission arises.
Steady-state and time-resolved luminescence spectra of NaYF4:Yb/Er upconversion nanoparticles in acetonitrile were measured using a FluoTime 300 time-resolved spectrometer, equipped with a 980 nm excitation laser (LDH-D-C-980). The laser was controlled by a PDL 828 driver, which can operate it in either CW, pulsed or burst mode, the latter being the most efficient excitation mode for luminescence decays in the µs range. The luminescence decay was detected at 540 nm. The lifetime value indicates from which electronic state the emission arises.
Right, the steady state emission spectrum of NaYF4:Yb/Er particles upon CW excitation at 980 nm is shown. It was acquired with a PMA-C-192M detector with an especially broad spectral range.
Observing upconversion in porphyrin followed by FRET to a ruthenium complex
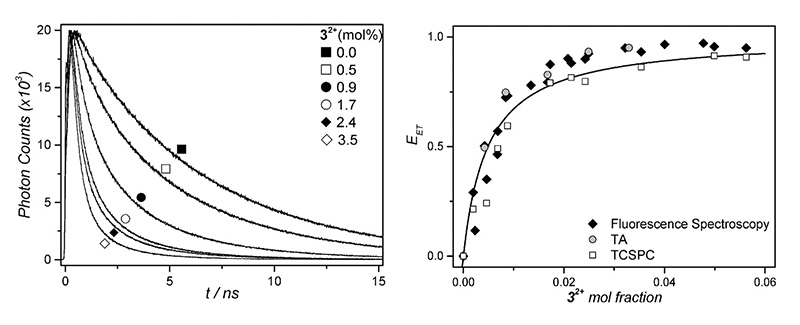 Photoactivatable anticancer therapy is a promising approach for hypoxic tumors. In order to work effectively inside tissues, photoactivation needs to be accomplished with wavelengths in the “phototherapeutic window” between 600 and 1000 nm. Upconversion offers the possibility to trigger photoactivation, which requires high-energy photons, with such NIR excitation light.
Photoactivatable anticancer therapy is a promising approach for hypoxic tumors. In order to work effectively inside tissues, photoactivation needs to be accomplished with wavelengths in the “phototherapeutic window” between 600 and 1000 nm. Upconversion offers the possibility to trigger photoactivation, which requires high-energy photons, with such NIR excitation light.
In this example, a porphyrine absorbs red light, next, triplet-triplet annihilation upconversion of perylene takes place, followed by non-radiative energy transfer to a ruthenium complex, which photodissociates.
Fluorescence decay curves were recorded in a FluoTime 200 time-resolved spectrometer featuring a 440 nm excitation laser. Reduction of the fluorescence lifetime proved non-radiative energy transfer to the ruthenium complex, and from the lifetimes, the transfer efficiencies can be calculated. Plotting the transfer efficiency versus the mole fraction and fitting with a modified Stern-Volmer curve yields the rate of quenching.
Read the paper in Physical Chemistry Chemical Physics to learn more: Triplet–triplet annihilation upconversion followed by FRET for the red light activation of a photodissociative ruthenium complex in liposomes
Characterization of TADF sensitizers for Green-to-UV upconversion
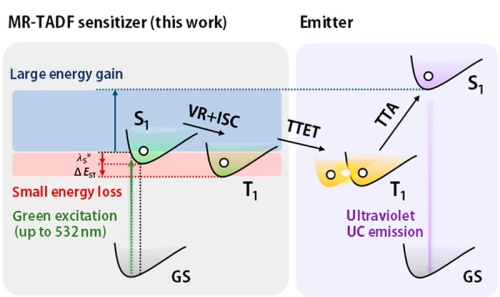 High-energy UV light is required for photopolymerization and photoligation reactions, for example. Instead of using expensive UV lasers, green light-emitting diodes could be used in combination with green-to-UV sensitizers capable of triplet-triplet annihilation upconversion. To minimize energy loss during triplet-triplet energy transfer (TTET) for triplet sensitization, thermally activated delayed fluorescence (TADF) molecules are employed here.
High-energy UV light is required for photopolymerization and photoligation reactions, for example. Instead of using expensive UV lasers, green light-emitting diodes could be used in combination with green-to-UV sensitizers capable of triplet-triplet annihilation upconversion. To minimize energy loss during triplet-triplet energy transfer (TTET) for triplet sensitization, thermally activated delayed fluorescence (TADF) molecules are employed here.
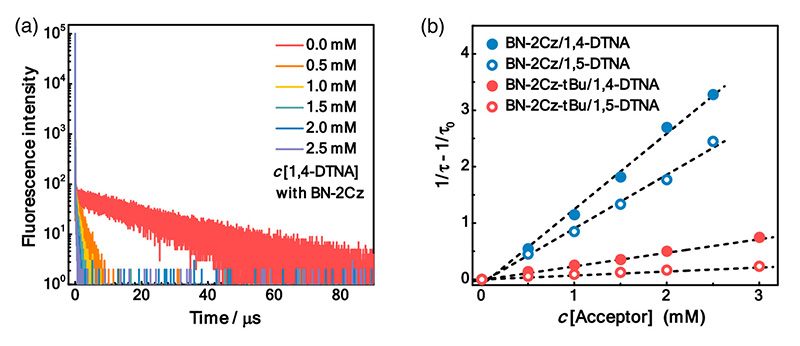
To characterize different sensitizer candidates, PL decay curves were acquired with a FluoTime 300 time-resolved spectrometer. They featured the double-exponential decay with a short ns lifetime and a long µs lifetime characteristic for TADF molecules. Quenching of the long component in the presence of oxygen showed that it originated from triplet states. The rates of inter-system crossing could be extracted from the data.
The energy transfer efficiency between sensitizer and acceptor can also be obtained and the influence of acceptor concentration studied.
Read the paper on ccs chemistry to learn more: Multiple Resonance Thermally Activated Delayed Fluorescence Sensitizers Enable Green-to-Ultraviolet Photon Upconversion: Application in Photochemical Transformations
 PicoQuant solutions to investigate upconversion materials:
PicoQuant solutions to investigate upconversion materials:
- Micro-photoluminescence Upgrade - Microscope-spectrometer coupling >
- FluoTime 300 - High-End Photoluminescence Spectrometer >
- FluoTime 250 - Compact Lifetime Fluorometer >
- MicroTime 100 - Compact Upright Photoluminescence Microscope >
- FluoMic - Compact Upright Widefield Photoluminescence Microscope >
- MicroTime 200 - Confocal Microscopic Platform >
- Wavelength Selection Unit FlexLambda >